A 15 Year Review (2006-2020) of Patient-Reported Outcome (PRO) in United States Oncology Product Labeling and Trends in Sponsor Size and Oncology Experience
Article Information
Lisa Cooper1*, Emily Wo2, Irene Lee2
1Department of Health Informatics, School of Health Professions, The State University of New Jersey, Newark, United States
2Ernest Mario School of Pharmacy, The State University of New Jersey, Newark, United States
*Corresponding author: Lisa Cooper, Stanley S. Bergen Building, Suite 136, Rutgers, The State University of New Jersey, 65 Bergen Street, Newark, NJ 07101-1709, United States
Received: 28 June 2022; Accepted: 14 July 2022; Published: 11 August 2022
Citation: Lisa Cooper, Emily Wo, Irene Lee. A 15 Year Review (2006-2020) of Patient-Reported Outcome (PRO) in United States Oncology Product Labeling and Trends in Sponsor Size and Oncology Experience. Archives of Internal Medicine Research 5 (2022): 412-419.
View / Download Pdf Share at FacebookAbstract
Objectives: Despite wide use of PRO tools in clinical development, resulting data is rarely incorporated into the US label. This study reviewed oncology product labels approved by the Food and Drug Administration (FDA) between 2006 and 2020 to determine if the number of PRO included in labeling has meaningfully changed. Sponsors were assessed to identify demographic trends in achieving PRO label success.
Methods: FDA-approved drugs were searched utilizing the Drugs@FDA database by month from January 2015 to December 2020 for novel drug and biologic approvals. Products approved between 2006-2014 were identified utilizing the Gnansakthy et al., 2012 and 2016 publications. Labels were reviewed for inclusion of PRO data in the label and product summary basis of approval (SBA). Sponsor size and experience were measured for each year of product approval.
Results: 155 oncology products received initial approval between 2006-2020, of which only 7 contained PRO data in the label. More than half (53.5%) of products had PRO data described in the SBA. Over time, PRO information increasingly been included in the product marketing application. Sponsors utilizing PRO data tend to be experienced in oncology development and larger in size.
Conclusions: There has been small increase in inclusion of PRO data in oncology product labeling over the past 15 years. Utilization and analysis of appropriate PRO tools and data remains a challenge to sponsors. Further collaboration with FDA is needed for the development of disease specific PRO tools that provide meaningful data to the targeted patient population.
Keywords
Patient-Reported Outcome, Oncology, Labeling
Article Details
1. Introduction
The use of patient-reported outcomes (PRO) in oncology drug development programs, and the ultimate translation of the resulting data into product labeling, has been extensively reviewed over the past 20 years. Despite the Food and Drug Administration’s (FDA) encouragement and guidance for industry members to prospectively collect PRO in a manner that may support treatment benefit claims, few approved oncology labels include such language [1-3].
In point, between 2006 and 2016, only 3 oncology product labels contained PRO statements, Zytiga®, Jakafi®, and Xalkori®, all of which were approved by FDA in 2011 [4-6]. The lack of PRO labeling in the US is attributed to several factors which have been documented consistently in the published literature [4-
8].
1.1 PRO tool/assessment measurements
To determine the appropriate PRO tool to utilize in an oncology development program, it is important to understand each tool’s purpose is. Quality of Life (QoL) tools are designed to measure the level of satisfaction of one’s life situation, which can include multiple items unrelated to the individual’s health status, such as financial security and relationship status. Therefore, such QoL tools are not acceptable to FDA for achieving labeling claims [7]. On the other hand, health-related quality of life (HRQoL) assessments focus solely on the individual’s health status and evaluate symptom measures which note the existence of the symptom, as well as determine the impact of the symptom(s) on patient function or well-being [9]. Hao (2010) classifies HRQoL tools into three distinct categories: 1) generic measures which look at a general population and may not be disease based, 2) general cancer measures that span cancer types, and 3) cancer-specific measures which are specific to a certain cancer type and its associated symptoms. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 (EORTC QLQC30), EuroQol 5-dimensionalquestionnaire (EQ-5D), and Functional Assessment of Cancer Therapy: General (FACT-G) are the most commonly used general cancer measurement tools observed in oncology clinical trials [6, 7, 9-12]. While the FDA does not specify which assessment tools to use, their 2009 Guidance for Industry for PRO outcome measures to support labeling claims does provide the characteristics they should contain [3].
Acceptability of PRO tools by health authorities varies. In fact, Gnanasakthy, et al. [6] found that between 2012-2016, nearly half of oncology product labels received PRO label language when data obtained by these tools was included in the market approval submission package to the European Medicines Agency (EMA), while no label language was granted by the FDA for the same products. Specifically, the EQ-5D, EORTC QLC-C30 with disease specific module, and FACT-G with disease specific module, had the highest number of inclusions in the EU label [6]. This difference in standards highlights the dynamic value-framework built to support each region’s health authority, payer, and prescriber requirements. In regions with single or national payer systems, it is not enough to simply get market approval, rather an evaluation of the overall cost effectiveness of a product is conducted to set price ceilings. In contrast, in regions where there is a private payer market such as the US, the health authority focuses on the product’s efficacy and safety while each independent payer separately evaluates overall value, and the level of evidence needed to influence payer decisions will vary [13].
1.2 Data interpretation
The FDA has stated that PRO instruments should be reliable, valid, and be able to detect change (i.e., sensitivity) [3]. Supplementary, Rock, et al. [7] note that interpretability of PRO data is reliant on three factors: 1) that the tool being used is capturing all relevant data; 2) that the results are consistent; and 3) that the results are clinically relevant to the population being studied even if statistically significant. Achieving these requirements is challenging as oncology patient populations are complex even within the same indication. As a result of this complexity, general assessment tools may not be sufficient to support label claims. To help mitigate this, disease specific modules have been developed, for example, EORTC QLC-C30 has roughly 60 disease specific questionnaires; however, not all such modules are validated. Additionally, most tools contain a multitude of measures or domains, and results may be positive in some domains, while negative or unchanged in others, challenging the robustness of findings [14]. Ultimately, the expectation is that the findings support the more conventional study endpoints (i.e., overall survival, overall response rate, duration of response, etc.). On the other hand, even if findings are statistically positive, if the changes are small, they may not be meaningful. One must also consider the potential for multiplicity when analyzing the data and ensure that the hypothesis being tested is well defined. Finally, studies which are not randomized or blinded have a high likelihood of confounding results due to patient bias [3, 7]. To combat several of these issues, FDA recommends developing an end point model which can be discussed with the agency during developmental meetings. Such models tie specified primary and secondary endpoints, their outcome measure(s) and assessment tool(s), to a clearly defined label claim [3]. The challenge here, is knowing what label content is desired early in the development program, which is often difficult [8].
1.3 Missing data
The FDA’s 2018 Guidance for Industry on oncology clinical trial endpoints clearly notes that “missing data and infrequent assessments can complicate the evaluation of symptom data particularly for time to deterioration analysis”. In addition, to demonstrate improvement of symptoms, patients must be symptomatic at baseline [15]. Despite this, missing data, including baseline data, is one of the primary reasons FDA rejects PRO data to support label claims [6, 7, 9, 10]. Oncology patients tend to have significant health complications and complex treatment plans which involve a high number of office visits and the length of time to complete a HRQL is an increased burden on the patient. Collection of baseline data also means that study sponsors must also have the PRO instrument in place at the time of study initiation, which is not always the case, especially for products which get approval based on earlier phase studies. Missing data must then be censored which may result in false results or inability to interpret outcomes. Methods to overcome missing data, such as missing completely at random (MCAR) and missing at random (MAR) analyses, as well as completing the HRQL by proxy present their own challenges and are not considered appropriate by FDA [7].
Not with standing the challenges collecting and analyzing PRO data can have, there are sponsors who do it properly and have achieved labeling claims in the US. The sponsors of the three products approved in 2011 with PRO label language, Zytiga, Jakafi, and Xalkori, include Janssen, Incyte Corporation, and Pfizer respectively. When it comes to new and costly ventures in oncology product development, many sponsors take a watch-and-wait approach, allowing other sponsors to work through the challenges and define a clear path to success. No previously published literature was found examining the sponsors who have successfully achieved PRO labeling, nor those who have failed. Previously published evaluations of oncology PRO labeling included labels approved up to 2016 [4-6]. The purpose of this study is to build upon the available published data by reviewing oncology product labels approved by the FDA between 2006 and 2020 to determine if the number of PRO inclusions in oncology labeling has meaningfully changed over this 15-year period and assess if the reasons for denial have remained consistent. In addition, product sponsors were appraised to identify the types of sponsors achieving PRO label success.
2. Methods
FDA-approved drugs were searched utilizing the Drugs@FDA database by month from January 2015 to December 2020 for novel drug and biologic approvals, were searched for oncology products, and then oncology indications were verified by a second investigator. Only products whose label “Initial U.S. Approval” date matched the NDA/BLA approval date within the database were included, eliminating older products which were noted as original submissions. Supplemental submissions and generic products were excluded. Oncology products approved between 2006-2014 were identified utilizing the Gnansakthy et al., 2012 and 2016 publications which followed the same methodology [4, 5]. Following identification, labels approved between 2015-2020 were searched for PRO inclusion, with particular focus on the Clinical Studies section of the package insert. Drug approval packages were reviewed for inclusion of PRO data application submitted to FDA for approval. Within the drug approval packages the Medical Review was examined when available, and in the absence of a Medical Review, the Multidisciplinary Review was utilized. In circumstances where PRO was included in the drug approval package but was not included in the product label, the FDA reviewer’s reason(s) for rejecting the data were documented. The PRO assessment tool was recorded for all PRO data found in the drug approval packages and label. Gnansakthy et al., 2012 and 2016 were utilized to identify PRO label content and PRO inclusion in summary approval packages for products approved between 2006-2014 [4, 5]. The type of FDA application review was noted for all products as follows: standard, priority, and/or accelerated. Finally, orphan drug status was also flagged.
Sponsor demographic data, including company size (number of employees) and the number of marketed oncology products the sponsor had at the time of product approval, was retrieved by searching the company product portfolios. The number of sponsor oncology products approved before or within the year of each product within the dataset was recorded. When needed, such as when a sponsor no longer exists, Wayback Machine (wayback.archive.org) was used to access archived company websites. Sponsors were then categorized as follows: (1) 0-2 products at time of approval [little experience], (2) 3-5 products at time of approval [moderate experience], and (3) 6 or more products at time of approval [experienced]. Google was used to retrieve the estimated number of employees for each company during the year of product approval. Sponsor size was then categorized as follows: (1) 1-499 employees [small], (2) 500-9,999 employees [medium], and (3) 10,000 employees or greater [large].
3. Results
The final dataset consisted of 155 unique oncology products approved between 2006-2020. Three products, Bavencio® (avelumab), approved in 2017, and Tazverik® (tazemetostat) and Gavreto® (pralsetinib), both approved in 2020, received approval under two separate applications; however, these products were only counted as a single unique product in the database. Over the 15-year review span, 7 products (4.5%) had labels containing PRO data. One product, Zirabev® (bevcizumab-bvzr) notes PRO as a secondary study outcome; however, no data or summary of results is provided. Therefore, Zirabev was not counted as having PRO in the label. Additionally, Xalkori (crizotinib) was noted by Gnanasakthy, et. al (2016) as having PRO in the label; however, the exact contribution of PRO data supporting label language, specifically visual safety events, could not be verified by this research group in the initial product label.
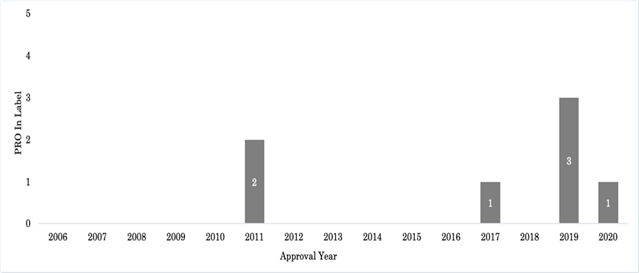
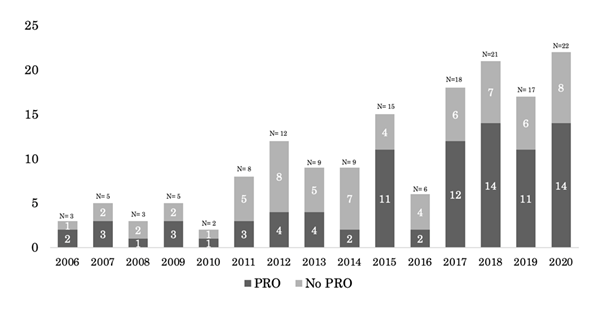
The Visual Symptom Assessment Questionnaire is mentioned as being added to the pivotal Xalkori study during an amendment, but no further discussion on the tool is provided with the drug approval package, and it is therefore difficult to determine what, if any, data is directly linked to the label content. The addition of PRO language was found in the Xalkori product label under the header “Description of selected adverse drug reactions: Vision disorders” in 2013, two years following initial approval; however, as this was a modification to the label post-initial approval, this product was not counted as having PRO at the time of initial approval. Two labels containing PRO were approved in 2011, one in 2017, three in 2019, and one label in 2020. Figure 1 shows approval of PRO containing labels over time from 2006-2020. In addition, PRO was reviewed as part of the submission pack and discussed in the product approval summary for 87 products (53.5%). The majority of products with PRO in the approval summary were approved between 2016-2020 (n=53/87; 60.9%). Inclusion of PRO data in the approval summary by year as a portion of all yearly approvals is presented in Figure 2.
As described in Table 1, three of the seven products that were granted PRO labeling, Rituxan Hycela®, Herceptin Hylecta™, and Phesgo™, utilized tools that measured preference for route of administration. The results are described under the “Patient Experience” subheading in the clinical studies section of each of the three labels. For these products, patients were asked to complete a preference questionnaire post-treatment to document their preference for subcutaneous or intravenous administration and their reasoning. Two of the seven products, Zytiga and Nubeqa™, used the Brief Pain Inventory-Short Form, which is designed to assess the severity of pain in chronically ill or cancer patients and the impact of pain on their daily functioning. PRO measurement tools specific to the disease indication were implemented for Jakafi and Inrebic®, both indicated for myelofibrosis, which used the Myelofibrosis Symptom Assessment Form version 2.0 to measure changes in the patient’s Total Symptom Score. This tool allows patients to report on the six core symptoms of myelofibrosis (night sweats, itching, abdominal discomfort, early satiety, pain under ribs on left side, and bone or muscle pain). These data were included as additional clinical endpoints for the clinical studies for both products.
Across the 87 oncology products with PRO language in the approval packages, a total of 168 PRO tools were cited. The most common PRO tools included EORTC QLQC30 (37/168; 22%), Functional Assessment of Cancer Therapy for multiple indications (20/168; 11.9%), EuroQol 5-dimensional questionnaire (EQ-5D) (14/168; 8.3%), EQ-5D-5L-an update to EQ-5D (11/168; 6.5%), and EORTC QLQ-LC13 -a supplemental module to EORTC QLQ-LC13 for lung cancer (7/168; 4.2%). Thirty-five products utilized 1 tool, 28 products utilized 2 tools, 19 products utilized 3 tools, and 5 products utilized 4 tools. One hundred forty-four reasons were provided by FDA reviewers for refusal to include PRO in the product label, of which, the most cited reasons were: exploratory study (17/144; 11.8%), inadequate measurement/tools (14/144; 9.7%), missing data (12/144; 8.3%), open-label study (12/144; 8.3%), and single arm study (9/144; 6.3%). Of the products that the FDA provided denial reasons for, 57% (40/70) had more than one reason for PRO-labeling denial.
Of the seven products that incorporated PRO language in the product label at initial approval, three received standard approvals (Rituxan Hycela, Herceptin Hylecta, and Phesgo) and four received priority review (Zytiga, Jakafi, Inrebic, Nubeqa). None were approved under Accelerated Approval. Three were designated as orphan drugs, Jakafi, Rituxan Hycela, and Inrebic. For those products that did not have PRO label language (n=148), 108 (72.9%) were approved via priority review, 50 (33.8%) received accelerated approval, 39(26.4%) underwent standard approval, and 101 (68.2%) were designated as orphan indications. Of the products that had PRO in the approval package (n=87), 72 (82.8%) received priority review, 27 (31.0%) received accelerated approval, 15 (17.2%) received standard approval, and 61 (70.1%) were orphan designated. Conversely, for products with no PRO data in the approval package (n=68), 40 (58.8%) received priority approval, 23 (33.8%) received accelerated approval, 26 (38.2%) received standard approval, and 48 (70.6%) were orphan designated.
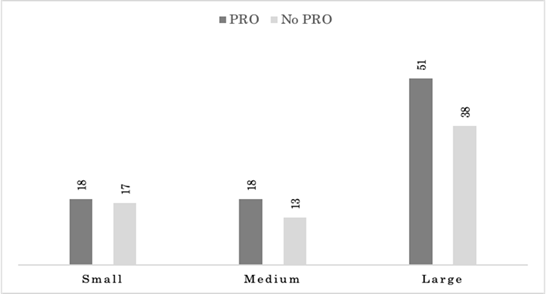
Overall, the majority of product sponsors (89/155; 57.4%) were large companies (10,000 or greater employees). The number of small- (1-499 employees) and medium- (500-9,999 employees) sized product sponsors were comparable (35/155; 22.6% and 31/155; 20%, respectively). Sponsors of products with PRO labeling were also large companies (5/7; 71.4%), with two (28.6%) medium-sized product sponsors and no small-sized sponsors. Sponsors of products which contained PRO information within the summary approval package tended to be large (51/87;58.6%), with medium (18/87; 20.7%) and small (18/87; 20.7%) equally represented. Sponsors of products whose approval summary did not contain PRO information were also most large companies (38/68; 55.9%), followed by small (17/68; 25%) and medium (13/68; 19.1). Figure 3 illustrates sponsor size by inclusion of PRO information in the summary approval package. For each company size category, companies that had PRO information in the approval summary were evenly split, 18/35 (51.4%) for small-, 18/31 (58.1) for medium-, and 51/89 (57.3%) for large-sized companies.
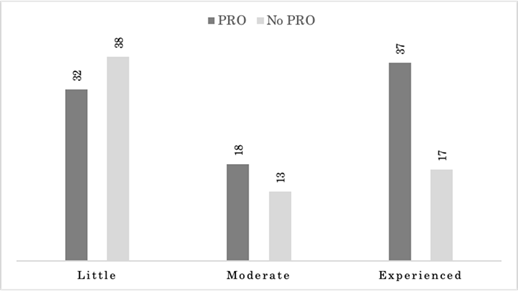
More product sponsors had little oncology experience (0-2 oncology products) at the time of product approval (70/155; 45.2%), compared to those that had moderate (3-5 products; 31/155; 20%), or were considered experienced (6 or more products; 54/155; 34.8%). Sponsors whose product contain PRO labeling were more often experienced (4/7; 57.1%), compared to moderately experienced (2/7; 28.6%), and those with little experience (1/7; 14.3%). Sponsors whose product approval summaries contained PRO information were led by experienced companies (37/87; 42.5%) followed by those with little experience (32/87; 36.8%), and finally moderate experience (18/87; 20.7%). Sponsors with little experience made up the majority of products that did not have PRO information in the approval summary (38/68; 55.9%). Experienced sponsors made up 25% (17/68) and moderately experienced sponsors comprised 19.1% (13/68) of the remaining products with no PRO information in the summary approval as shown in Figure 4. As a percentage of each experience type, those that had PRO information in the approval summary were primarily experienced sponsors (37/54; 68.5%), followed by moderately experienced (18/31; 58.1%), and little experience 32/70; 45.7%).
The total number of oncology labels containing patient reported outcome (PRO) data receiving initial market approval are depicted by year from 2006-2020.
The total number of summary basis of approvals for oncology products containing patient reported outcome (PRO) data receiving initial market approval are depicted by year from 2006-2020.
Product summary basis of approvals for approved oncology products between 2006-2020 are categorized sponsor size and whether they contain patient reported outcome (PRO) data or not. Sponsor size was determined by number of employees as follows: small (1-499 employees), medium (500-9,999 employees), and large (10,000 or greater employees).
|
Name |
Company |
Year Approved |
Indication |
PRO Model/Tool |
Label Language |
|
Zytiga (abiraterone acetate) |
Janssen |
2011 |
Prostate Cancer |
Brief Pain Inventory-Short Form |
45% of trial participants had a Brief Pain Inventory score of ≥ 4 at baseline. |
|
Jakafi (ruxolitinib) |
Incyte Corporation |
2011 |
Myelofibrosis |
Total Symptom Score: Myelofibrosis Symptom Assessment Form (MFSAF) v2.0 diary |
Secondary endpoints included the proportion of patients with a 50% or greater reduction in Total Symptom Score from baseline to week 24, measured by the modified Myelofibrosis Symptom Assessment Form (MFSAF) v2.0 diary. |
|
Rituxan Hycela (rituximab and hyaluronidase human) |
Genentech |
2017 |
Follicular Lymphoma, |
Preference for route of administration |
77% of patients reported preferring subcutaneous administration over intravenous rituximab due to less time spent in the clinic. 11% preferred rituximab intravenous administration due to more comfortable administration. (7.7%) had no preference. (4.7%) did not complete the preference questionnaire. |
|
Herceptin Hylecta (trastuzumab and hyaluronidase-oysk) |
Genentech |
2019 |
HER-2 + Breast |
Preference for route of administration |
86% of patients reported preferring subcutaneous administration over intravenous administration due to less time spent in the clinic. 13% reported preferring intravenous treatment due to fewer local injection reactions. 1% had no preference for the route of administration. 3.8% withdrew from the treatment before completing the survey. |
|
Inrebic (fedratinib) |
Celgene |
2019 |
Myelofibrosis |
Total Symptom Score: Myelofibrosis Symptom Assessment Form (MFSAF) v2.0 diary |
The modified MFSAF v2.0 is a patient diary capturing the 6 core symptoms of MF completed daily. One of the trial outcomes was the proportion of patients with a 50% or greater reduction in Total Symptom Score from baseline to the End of Cycle 6 as measured by MFSAF. The proportion of patients with a 50% or greater reduction in Total Symptom Score was 40% in the treatment arm and 9% in the placebo arm. |
|
Nubeqa (darolutamide) |
Bayer |
2019 |
Prostate Cancer |
Brief Pain Inventory-Short Form |
Baseline pain was measured using the Brief Pain Inventory, at baseline 47% of patients had no pain. |
|
Phesgo (pertuzumab, trastuzumab, and hyaluronidase-zzxf) |
Genentech |
2020 |
HER-2 + Breast Cancer |
Preference for route of administration |
85% of patient reported preferring subcutaneous administration over intravenous due to less time spent in the clinic. 14% reported preferring intravenous administration due to more comfortable administration. 1% had no preference. |
Table 1: Initial oncology product approvals containing labeled patient reported outcome (PRO) data and their corresponding PRO tools.
Product summary basis of approvals for approved oncology products between 2006-2020 are categorized sponsor experience and whether they contain patient reported outcome (PRO) data or not. Sponsor experience was determined by number of oncology products approved each year as follows: little experience (0-2 products at time of approval), moderate experience (3-5 products at time of approval), and experienced (6 or more products at time of approval).
4. Discussion
This review reveals the trends in PRO inclusion in oncology development and product labeling over a 15-year time period, from 2006 to 2020. Inclusion of PRO in development programs and evaluated as part of the marketing application submission package by FDA has hovered around 50% of initial product submissions, with an approximate 10% increase in the last 5 years. However, this increase of PRO information in the product approval packages has not translated into more PRO product labeling. Furthermore, although use of PRO tools in oncology clinical trials appear to be common, and their utilization is generally to provide information regarding quality of life or health-related quality of life, 3 of the 7 oncology products that received PRO labeling were for preference of route of administration (Rituxan Hycela, Phesgo, Herceptin Hycela). These were the only 3 instances where preference for route of administration was utilized during the 15-year time span. Notably, Rituxan Hycela, Phesgo, Herceptin Hycela each conducted separate clinical studies to evaluate patient preference for route of administration, and in each of these clinical studies PRO for route of administration was a primary objective or a primary endpoint. This is notable, as the most common reason for FDA denial of PRO inclusion in product labeling was due to the exploratory nature of these endpoints.
The FDA analysis of PRO label inclusion for Phesgo stated that, “The results of the patient preference questionnaire benefit from a reasonable study design, straightforward instrument, and high completion rate…”. [16]. Of the four remaining product labels containing PRO, two utilized the Brief Pain Inventory-Short Form and two utilized the Total Symptom Score for myelofibrosis (MFSAF). As the majority of PRO tools described in the approval packages included generic assessments such as the EORTC QLQC30, Functional Assessment of Cancer Therapy for multiple indications, and EQ-5D, it remains clear that the FDA still requires a definitive link between the PRO tool measurements and the indication studied. Sponsors appear to continue to struggle to find and appropriately implement the right tool for their specific tumor type within their development programs. These findings remain consistent with the Gnanasakthy, et. al. (2016) study, which recognized similar limitations to PRO inclusion, such as open-label study design and lack of disease-specific tools [5].
Most products received priority approval (114/155; 73.5%), and about a third (50/155; 32.3%) received accelerated approval. It may therefore be reasonable to consider that complete PRO data may not be available at the time of submission of the marketing application, thereby rendering its inclusion into the product label unlikely. Upon review, this explanation is unfounded, as 4 of the 7 products with PRO in the label received priority review. Additionally, the percent of products with standard approval which did not contain PRO in the approval package was nearly twice that of products with standard approval which did contain PRO in the approval package. Accelerated approval did not appear to have any bearing on inclusion of PRO or not. Sponsor size and experience in the oncology field were considered as possible influences on acceptance of PRO language in the product label, as one may expect that sponsors with greater resources and experience would have a larger number of products incorporating PRO adequately in each development program. Indeed, Rituxan Hycela, Phesgo, Herceptin Hycela were all sponsored by Genentech, a large biotechnology company with considerable experience with oncology products. Additionally, Nubequ and Zytiga, sponsored by Bayer and Janssen, reflect PRO product labeling by large companies. This is further supported by the findings that PRO inclusion in the summary package is led by more experienced sponsors, while those that did not contain PRO were headed by sponsors with little experience. While sponsor size did not vary meaningfully between PRO inclusion or exclusion in the summary package, sponsor disposition cannot be excluded as a factor in the appropriate use and acceptance of PRO data supporting the product label.
This study was limited to reviewing labels of initial product approvals, and it is acknowledged that some labels may have received inclusion of PRO language in a subsequent label update. This may particularly be true for those products which received approval through accelerated development and approval methods. One example of a subsequent approval resulting in PRO language is Imbruvica (ibrutinib), which received initial approval in 2013 for mantle cell lymphoma (MCL). The drug was subsequently approved in 2017 for graft versus host disease (GVHD), and data from the Lee Symptom Scale (LLS) PRO tool, which was used as a secondary endpoint, was included in the label as the FDA “review team decided that information from the LLS would be helpful for the practitioner as LSS is used as part of the cGVHD assessment at patient visits” [17].
5. Conclusions
Overall, inclusion of PRO tools in clinical programs for oncology products has increased over the past 15 years; however, this has not translated into an increased number of products with PRO language in the product label. Sponsors continue to struggle with finding the tool most appropriate for their indication as well as appropriately including those tools in a statistically meaningful manner at the right time in the development program. Sponsors continue to utilize common QoL or HRQoL tools accepted by ex-US health authorities, but which are repeatedly rejected by FDA. The continued lack of PRO labeling in the US highlights the need for further direction from the FDA as well as collaborative development of disease specific PRO tools.
Funding and Acknowledgements
Research reported in this publication was supported by the National Center for Advancing Translational Sciences (NCATS), a component of the National Institute of Health (NIH) under award number UL1TR003017. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors declare no financial or other conflicts of interest related to this work.
References
- Johnson JR, Temple R. Food and Drug Administration requirements for approval of new anticancer drugs. Cancer Treat Rep 69 (1985): 1155-1159.
- Food and Drug Administration. Human Subject Protection-Information for Institutional Review Boards, Clinical Investigators, and Sponsors; Rescission, Reissuance, and Development of Food and Drug Administration Guidance Documents (2006).
- Center for Drug Evaluation and Research. Patient-Reported Outcome Measures: Use in Medical Product Development. U.S. Food and Drug Administration (2009).
- Gnanasakthy A, Mordin M, Clark M, et al. A review of patient-reported outcome labels in the United States: 2006 to 2010. Value Health 15 (2012): 437-442.
- Gnanasakthy A, DeMuro C, Clark M, et al. Patient-Reported Outcomes Labeling for Products Approved by the Office of Hematology and Oncology Products of the US Food and Drug Administration (2010-2014). J Clin Oncol 34 (2016): 1928-1934.
- Gnanasakthy A, Barrett A, Evans E, et al. A Review of Patient-Reported Outcomes Labeling for Oncology Drugs Approved by the FDA and the EMA (2012-2016). Value Health 22 (2019): 203-209.
- Rock EP, Scott JA, Kennedy DL, et al. Challenges to use of health-related quality of life for Food and Drug Administration approval of anticancer products. J Natl Cancer Inst Monogr (2007): 27-30.
- Shields AL, Hao Y, Krohe M, et al. Patient-Reported Outcomes in Oncology Drug Labeling in the United States: A Framework for Navigating Early Challenges. Am Health Drug Benefits 9 (2016): 188-197.
- Hao Y. Patient-reported outcomes in support of oncology product labeling claims: regulatory context and challenges. Expert Rev Pharmacoecon Outcomes Res 10 (2010): 407-420.
- Safa H, Tamil M, Spiess PE, et al. Patient-Reported Outcomes in Clinical Trials Leading to Cancer Immunotherapy Drug Approvals From 2011 to 2018: A Systematic Review. JNCI: Journal of the National Cancer Institute 113 (2020): 532-542.
- Hao Y, Krohe M, Yaworsky A, et al. Clinical Trial Patient-reported Outcomes Data: Going Beyond the Label in Oncology. Clinical therapeutics 38 (2016): 811-820.
- Gondek K, Sagnier PP, Gilchrist K, et al. Current status of patient-reported outcomes in industry-sponsored oncology clinical trials and product labels. J Clin Oncol 25 (2007): 5087-5093.
- Brogan AP, DeMuro C, Barrett AM, et al. Payer Perspectives on Patient-Reported Outcomes in Health Care Decision Making: Oncology Examples. Journal of Managed Care & Specialty Pharmacy 23 (2017): 125-134.
- Questionnaires: EORTC – Quality of Life. EORTC (2019).
- Oncology Center of Excellence. Clinical Trial Endpoints for Approval of Cancer Drugs and Biologics. U.S. Food and Drug Administration (2018).
- US Food and Drug Administration. Multi-Discipline Review. Drug Approval Package for Phesgo (2020).
- US Food and Drug Administration. Approval Package for Imbruvica (2022).