Using Nanoparticles and Laser Induced Photo Thermal Ablation to Treat Low Grade Canine Mast Cell Tumors: Evaluation of Efficacy and Safety
Article Information
Lisa Parshley1*, Lisa Miller2, Luis De Taboada2, Chelsea Tripp3, Scott Gustafson1, Emily Mouat1, Abbey Bradley1, Tammy Melton1, Evan Pape1, Shane Sitzman1
1Olympia Veterinary Specialists - the Cancer Center, 115 Eastside Street NE, Olympia, 98506, WA, USA
2Companion Animal Health, 101 Lukens Dr, Suite A, New Castle, 19720, DE, USA
3Bridge Animal Referral Hospital, 8401 Main St, Edmonds, 98026, WA, USA
*Corresponding Author: Lisa Parshley, Olympia Veterinary Specialists - the Cancer Center, 115 Eastside Street NE, Olympia, 98506, WA, USA.
Received: 04 April 2022; Accepted: 18 April 2022; Published: 28 April 2022
Citation: Lisa Parshley, Lisa Miller, Luis De Taboada, Chelsea Tripp, Scott Gustafson, Emily Mouat, Abbey Bradley, Tammy Melton, Evan Pape, Shane Sitzman. Using Nanoparticles and Laser Induced Photothermal Ablation to Treat Low Grade Canine Mast Cell Tumors: Evaluation of Efficacy and Safety. Journal of Nanotechnology Research 4 (2022): 45-58
View / Download Pdf Share at FacebookAbstract
Background: Nanoparticles have been the subject of a large amount of physical and bioscience research. In the last decade use of these particles in medicine has gone from theoretical to clinical trials. Passive targeting of certain nanoparticles takes advantage of inherent abnormalities in tumor vasculature allowing accumulation in solid tumors through a process known as the ‘‘Enhanced Permeability and Retention’’ (EPR) effect. In animal tumor implant models, Gold-Coated Silicone Nanoparticle (GSN) and exposure of tumors to laser light (at 808nm) generated enough heat to cause tumor cell death. Mast Cell Tumors (MCT) are the most common skin tumor in dogs, comprising an estimated 20% of canine skin tumors. The goal of this retrospective study was to evaluate nanoparticle and laser Photothermal Ablation (PTA) on low grade canine MCT.
Results: 30 dogs with 36 mast cell tumors were treated in this retrospective study. All tumors were low grade MCT based on histopathologic examination. Treated dogs had a 100% response rate, with 94% achieving clinical remission. Recurrence rate was 17%. Mean Progression Free Time (PFT) for treated dogs was 552 days.
Conclusion: Results of this retrospective study suggest that photothermal ablation using GSN combined with exposure to near infrared light (808 nm) may provide an effective local therapy of low-grade canine MCT. Median progression free time and survival was not reached in the treatment group, suggesting that long term tumor control may be possible with PTA that potentially equals surgery when margins are narrow (<0.3cm) or incomplete.
Keywords
Cancer; Canine; Laser; Mast Cell Tumor; Nanoparticles; Photothermal Ablation
Cancer articles Cancer Research articles Cancer review articles Cancer PubMed articles Cancer PubMed Central articles Cancer 2023 articles Cancer 2024 articles Cancer Scopus articles Cancer impact factor journals Cancer Scopus journals Cancer PubMed journals Cancer medical journals Cancer free journals Cancer best journals Cancer top journals Cancer free medical journals Cancer famous journals Cancer Google Scholar indexed journals Canine articles Canine Research articles Canine review articles Canine PubMed articles Canine PubMed Central articles Canine 2023 articles Canine 2024 articles Canine Scopus articles Canine impact factor journals Canine Scopus journals Canine PubMed journals Canine medical journals Canine free journals Canine best journals Canine top journals Canine free medical journals Canine famous journals Canine Google Scholar indexed journals Laser articles Laser Research articles Laser review articles Laser PubMed articles Laser PubMed Central articles Laser 2023 articles Laser 2024 articles Laser Scopus articles Laser impact factor journals Laser Scopus journals Laser PubMed journals Laser medical journals Laser free journals Laser best journals Laser top journals Laser free medical journals Laser famous journals Laser Google Scholar indexed journals Mast Cell Tumor articles Mast Cell Tumor Research articles Mast Cell Tumor review articles Mast Cell Tumor PubMed articles Mast Cell Tumor PubMed Central articles Mast Cell Tumor 2023 articles Mast Cell Tumor 2024 articles Mast Cell Tumor Scopus articles Mast Cell Tumor impact factor journals Mast Cell Tumor Scopus journals Mast Cell Tumor PubMed journals Mast Cell Tumor medical journals Mast Cell Tumor free journals Mast Cell Tumor best journals Mast Cell Tumor top journals Mast Cell Tumor free medical journals Mast Cell Tumor famous journals Mast Cell Tumor Google Scholar indexed journals Nanoparticles articles Nanoparticles Research articles Nanoparticles review articles Nanoparticles PubMed articles Nanoparticles PubMed Central articles Nanoparticles 2023 articles Nanoparticles 2024 articles Nanoparticles Scopus articles Nanoparticles impact factor journals Nanoparticles Scopus journals Nanoparticles PubMed journals Nanoparticles medical journals Nanoparticles free journals Nanoparticles best journals Nanoparticles top journals Nanoparticles free medical journals Nanoparticles famous journals Nanoparticles Google Scholar indexed journals Photothermal Ablation articles Photothermal Ablation Research articles Photothermal Ablation review articles Photothermal Ablation PubMed articles Photothermal Ablation PubMed Central articles Photothermal Ablation 2023 articles Photothermal Ablation 2024 articles Photothermal Ablation Scopus articles Photothermal Ablation impact factor journals Photothermal Ablation Scopus journals Photothermal Ablation PubMed journals Photothermal Ablation medical journals Photothermal Ablation free journals Photothermal Ablation best journals Photothermal Ablation top journals Photothermal Ablation free medical journals Photothermal Ablation famous journals Photothermal Ablation Google Scholar indexed journals therapy articles therapy Research articles therapy review articles therapy PubMed articles therapy PubMed Central articles therapy 2023 articles therapy 2024 articles therapy Scopus articles therapy impact factor journals therapy Scopus journals therapy PubMed journals therapy medical journals therapy free journals therapy best journals therapy top journals therapy free medical journals therapy famous journals therapy Google Scholar indexed journals Photothermal articles Photothermal Research articles Photothermal review articles Photothermal PubMed articles Photothermal PubMed Central articles Photothermal 2023 articles Photothermal 2024 articles Photothermal Scopus articles Photothermal impact factor journals Photothermal Scopus journals Photothermal PubMed journals Photothermal medical journals Photothermal free journals Photothermal best journals Photothermal top journals Photothermal free medical journals Photothermal famous journals Photothermal Google Scholar indexed journals cells articles cells Research articles cells review articles cells PubMed articles cells PubMed Central articles cells 2023 articles cells 2024 articles cells Scopus articles cells impact factor journals cells Scopus journals cells PubMed journals cells medical journals cells free journals cells best journals cells top journals cells free medical journals cells famous journals cells Google Scholar indexed journals tumor vasculature articles tumor vasculature Research articles tumor vasculature review articles tumor vasculature PubMed articles tumor vasculature PubMed Central articles tumor vasculature 2023 articles tumor vasculature 2024 articles tumor vasculature Scopus articles tumor vasculature impact factor journals tumor vasculature Scopus journals tumor vasculature PubMed journals tumor vasculature medical journals tumor vasculature free journals tumor vasculature best journals tumor vasculature top journals tumor vasculature free medical journals tumor vasculature famous journals tumor vasculature Google Scholar indexed journals
Article Details
Abbreviations:
EPR: Enhanced Permeability and Retention; GSN: Gold and Silicone Nanoparticle; PEG: Polyethylene Glycol; NIR: Near infrared radiation; MCT: Mast cell tumors; RT: Radiation Therapy; PTA: Photothermal Ablation; PFT: Progression Free Time; RR: Response Rate; CR: Clinical Remission; PR: Partial Remission
1. Introduction
Nanoparticles for over forty years have been the subject of a large number of physical and bioscience research [1]. In the last decade use of these particles in medicine has gone from theoretical to clinical trials [2]. Current biomedical research now suggests nanoparticles will in the near future provide cancer patients with targeted drug delivery, manipulation of cancer microenvironment, provide radio sensitization and chemo sensitization, aid in immunotherapy, and are the basis for new methods in bioimaging [3-19]. These tiny particles get their name from their size, ranging from 1 nm to several hundred nm. Construction occurs at the molecular level using chemical interactions between different compounds and elements [19-22]. Based on their size, nanoparticles are, in fact, well matched to interact with biological molecules found inside and outside cells. For example, mammalian cells range in size from 2000 nm to 10,000 nm, cellular organelles are about 100 nm to 300 nm, and intracellular proteins and molecules range between 10 nm to 50 nm [23]. Nanoparticles have several attributes that support their use in biomedical research and medical therapy at a microscopic level. First is their large surface area to volume ratio, allowing significantly increased chemical reactions to occur on their surface [19]. A second property is that they are often an appropriate size for intravascular conveyance and targeted accumulation in tumors [19, 24, 25]. These properties among many others give nanoparticles a potential to target and manipulate tissues at a molecular level [23]. The specific compounds or elements used to make a nanoparticle define how they can be employed in medical or cancer therapy and/or radiology [1-3, 9, 19-21]. Nanoparticles are most often constructed using more than one compound, such as silicone and metals or lipids and glycols. Biologic behavior of a nanoparticle is dependent upon its molecular nature. Targeting of nanoparticles to specific tissues, such as cancer, is through active or passive mechanisms [1-3, 11, 19]. For example, attaching a ligand to the surface of a nanoparticle will actively target it to specific cells through cell surface receptor interactions, such as transferrin or folate receptors. Nanoparticles can also be targeted by attaching monoclonal antibodies or small molecules [1, 12, 24].
Nanoparticles that contain elements or metals, such as gold, can be used to enhance radiographic imaging or be used to induce vibration with exposure to appropriate wavelengths of light. Metal-based nanoparticles in combination with laser produced light are already in pilot studies or clinical trials. Early data on this combination suggests that it is possible to perform targeted heating of tumors achieving both coagulation of tumor vasculature and heat induced apoptosis or necrosis [1-4, 6, 9, 14, 17, 19, 25]. Passive targeting of metal-based nanoparticles takes advantage of inherent abnormalities in tumor vasculature (Figure 1) allowing accumulation in solid tumors through a process known as the ‘‘Enhanced Permeability and Retention’’ (EPR) effect.26 For example, a company in Houston, Texas created a Gold-Coated Silicone Nanoparticle (GSN) with a core of silicone and a shell of gold. Over the surface of the gold is an outer layer of Polyethylene Glycol (PEG) (Nanospectra Biosciences). By including the PEG layer, GSN has an increased circulation time by reducing mononuclear phagocytic system clearance [27]. After passive accumulation in tumors, exposure wavelength of near infrared light (808nm) causes resonance of gold atoms in the GSN shell. Reverberation of nanoparticles within the tumors causes conversion of light energy into heat energy [25, 28].
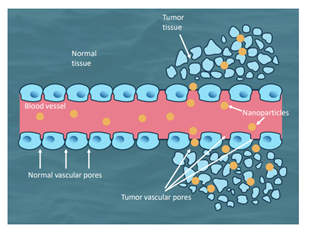
Figure 1: Graphic demonstration on passive accumulation of nanoparticles through abnormal tumor vasculature and not normal vasculature.
Previous research employing animal models has established that these inert GSNs are non-toxic, even at high doses. At a microscopic level there have been no toxicities noted in various organs, including the liver and spleen, the two organs responsible for clearing GSN from circulation that have not accumulated within tumors [29]. In animal tumor implant models, combination of GSN and exposure of tumors to laser light (at 808nm) generated enough heat to cause tumor cell death [14]. There has been initial pilot study data published in various canine tumor types [4,30] as well as ongoing human clinical trials in prostate cancer [8-10] and recurrent or refractory head and neck cancers (clinicaltrials.gov NCT00848042). Transcutaneously applied laser light undergoes loss of energy as it is transmitted through intervening biological tissues. Thus, interstitial laser fibers are employed to achieve nanoparticle PTA for internal tumors. External transcutaneous light application will limit this therapy to superficially accessible tumors/tissues [26]. Mast cell tumors are the most common skin tumor in dogs, with an estimate of MCT being roughly 20% of canine skin tumors [31-34]. Dogs may have solitary or multiple tumors at time of diagnosis, with dogs who form one tumor being more likely to form additional tumors [33]. They can be located anywhere on the body but are most common in the dermis [31-35]. Genetically, any breed can be affected but there are certain breeds predisposed to forming these tumors including Boston Terriers, Pitbulls, Pugs, Labrador Retrievers, and Boxers [31-35]. A majority of MCT occur in the caudal half of the body with a large percentage of these affecting limbs, the tail, and feet. These locations present challenges for complete surgical resection without amputation or extensive skin flaps [33, 35]. Prognostic factors for MCT in dogs are controversial. Factors that appear to impact long term control of the disease include location of the tumor, ability to perform adequate surgical removal, surgical margins, number of tumors at time of diagnosis, grade (both Kiupel and Patnaik grading schemes) [36, 37], subcutaneous tumors, histologic pattern, multinucleation, Ki67, Ki67 + AgNOR, and KIT cellular localization pattern, and stage. Several of these prognostic factors have opposing reported data suggesting substantially different outcomes. For example, controversy exists over the impact of surgical margins and what defines an adequate margin [38,39-40]. No consensus has been reached on what recurrence rate can be expected for completely or incompletely resected tumors. In the literature, recurrence rates range from 0 - 37.5% for clean margins and 12-63% for incompletely or narrowly resected (<3 mm margins) tumors. When factoring in grade, high grade (or high grade II or Grade III Patnaik) even with reasonable margins (>20 mm) have a 40% recurrence rate [31-37, 41-46]. It has been mostly accepted that grade of the MCT and surgical margins achieved during resection will impact long term tumor control [30-37, 41-44]. Regardless of the prognostic value of surgical margins, a majority of MCT can be cured or have long term control with suitable local therapy [31-33] Local therapies, other than surgery, are limited to Radiation Therapy (RT) and Electro chemotherapy (ECT) [32, 33, 47-52]. Radiation therapy has been suggested to reduce recurrence rates after incomplete surgical margins [48] and may be able to act as sole local therapy [9-51] in combination with standard common adjunctive systemic therapies (antihistamines and steroids). Electro chemotherapy as an adjunctive treatment for incompletely resected MCT and as a sole local therapy has intriguing support in the literature [47, 52]. Despite these two local therapies’ proven efficacy and durability of response, surgical resection is still considered standard of care for canine MCT. However, one recent study showed that MCT location was significantly associated with incisional complications following surgery, specifically, tumors located on the limbs were more likely to have incisional complications than tumors in other locations examined [53]. Therapeutic use of combined GSN and PTA on low grade canine MCT may provide an additional new local therapy against MCT, and possibly in the future, other superficial tumors. PTA may be especially useful in MCT locations that are not amenable to achieving complete surgical removal or when concerns for incisional complications exist. The purpose of this retrospective study was to examine the effect that PTA using GSN and near infrared light exposure had on canine low-grade mast cell tumors treated at two veterinary oncology hospitals over a four-year period. We hypothesized that this therapy provides a clinical effect and is well tolerated.
2. Materials and Methods
This retrospective multi-center study was aimed at assessing the response and progression free time of canine patients with low-grade MCT treated with GSN and laser PTA during the period from January 2016 through August 2020 at two veterinary oncology centers. Informed consent was obtained from pet owners for treatment. Since this was a retrospective study, no approval from an Ethical Committee was required. Decisions regarding whether to perform PTA were made according to each clinician’s discretion after surgery had been declined by the pet owners. Medical records were reviewed to identify dogs with first-occurring or recurrent low-grade or suspected low-grade dermal or subcutaneous MCT.
2.1. Patients Selection and Eligibility
To be eligible for data inclusion, dogs had to have undergone staging and treatment with GSN and PTA with a minimum follow-up of 365 days. Information on clinical staging was obtained by means of physical examination, abdominal ultrasound, and hematological and biochemistry analysis. Dogs with tumors that had any bruising or ulceration, that had been present for more than two months, that were histopathologically high-grade (Kiupel two grade system) MCTs or those with stage II-IV disease were excluded from the study. Also, dogs were excluded if they were receiving other neoadjuvant or adjuvant antitumoral treatment.
2.2. Data Collection and Analysis
Background information recorded for each dog included: signalment, MCT description (location and size) as well as description of any other dermal or subcutaneous masses, date of treatment, response, local relapse (defined as the presence of cytological evidence of a recurrent MCT within 2 cm from previous scar), any toxicities noted, date of death or last follow-up examination, and cause of death. All tumors were as considered individual data points for analysis. The characteristics of any local relapse and the survival impact was reported. All dogs were monitored post-treatment by means of clinical examination performed weekly for four weeks, then monthly during the first year, and every 6 months thereafter. At each appointment, the following information was recorded; mass size (using metric calipers), pictures of the masses, and information from the pet owner on how patients were doing at home was gathered. Clinical remission, partial remission, stable disease, and progressive disease were determined in accordance with previously published VOCG consensus for these designations in solid tumors [54]. Simple response rate, confidence ratio, mean progression free time, mean survival time, mean of time to response, and Kaplan-Meier survival curves evaluating progression free period, as well as median survival were calculated using Microsoft Excel spread sheet and MedCalc®. Kaplan Meier generated data was evaluated.
2.3. Clinical Treatment
All dogs treated with GSN and PTA were placed on diphenhydramine (2mg/kg PO q 12 hours) and famotidine (0.5mg/kg PO q 24 hours). Medications were administered at home for at least a week prior to infusion of nanoparticles and laser ablation. GSN nanoparticles used have a 120 nm diameter - silicone core and gold shell. A polyethylene glycol (PEG) outer layer gives the nanoparticle a total diameter of 150 nm. All dogs had intravenous (IV) infusions of GSN (5.2ml/kg) according to manufacturer§ instructions while monitoring temperature, heart rate, and respiratory rate. Infusions were via a peripheral IV catheter, a standard IV drip set utilizing a drip chamber, and an inline TNA-1 filter of > 5 microns (Pall Medical). Twenty-four (24) hours after infusion dogs were evaluated for any changes in the sentinel mass and had their tumors measured, shaved and cleansed with chlorhexidine surgical scrub and 0.9% saline. A local anesthetic ring block was performed using bupivacaine and lidocaine (3:1 ratio) with an addition of 10% of the total volume of sodium bicarbonate. Lidocaine hydrochloride topical solution USP (Viscous) 2% was applied directly to the area of the tumor to be exposed to light.
Laser safe eyewear was placed over the eyes of the treated dogs. All persons in the treatment area wore laser safe eyewear during the procedure. The laser device≥ was set to deliver 808 nm (±10nm) light via a specialized sapphire-tipped laser handpiece. This handpiece was designed to deliver a uniform irradiance beam (with a fixed laser spot area of 0.5cm2) to the surface of the tumor, and efficiently wick away surface heating generated by superficial tissue absorption (Companion Animal Health). Using the specialized laser handpiece, light was apply directly to the tumor surface. Light treatments ranged in radiant power from 3W up to 7W (resulting in an irradiance of 6-14W/cm2) depending on the size of the tumor. The number of light exposure sites per tumor varied between tumors, primarily to ensure the entire tumor’s exposed surface was treated. Transcutaneous laser parameters were selected according to manufacturer (Companion Animal Health) recommendations and based on the use of a predictive physics-based computational model (COMSOL Multiphysics®) of light diffusion in tissue and previous pilot studies in dogs and humans [4, 8, 9, 30]. Modeling helped predict increases in tissue temperature due to photon absorption and the presence and distribution of GSN’s within the tumors after intravenous administration. A thermal camera (Seek Thermal Camera) was used at 20-30 second intervals during laser treatment to ensure each treatment site reached appropriate temperature (ideally 55-65°C) and remained in this range for at least 5 minutes overall per tumor treated. If the treated site temperature did not reach ≥50°C after 1 minute of exposure, the power setting was increased incrementally by 0.5 W until appropriate temperature was reached. The maximum power setting did not exceed 7W. The total treatment time and maximum temperature reached was recorded for each laser application in seconds. Patients were recovered from anesthesia/sedation and monitored during recovery. Patients were sent home the same day as the laser procedure and were instructed to prevent licking or chewing through the use of physical barriers (e-collars) where applicable.
3. Results
3.1. Patients and Tumor Characteristics
30 dogs with 36 mast cell tumors were included in the analysis. The most represented breeds were mixed breed (n = 7, 23.3%) and Pitbull dogs (n = 6, 20%). Of the remaining dogs, 3 were Labrador Retrievers, 3 were Boxers, and 11 were breeds that were represented once or twice. Female dogs (n=19, 63.3%) outnumbered males (n=11, 36.5%). All dogs were neutered or spayed (Table 1).
|
Breeds |
Mixed (7), Pitbull (6), Labradors (3), Boxers (3), Terriers (2), Boston Terrier (2), Pug (2), Lhasa Apso (1), French bull dog (1), Pomeranian (1), New found land (1), Maltese (1) |
|
Female spayed |
19 |
|
Male |
11 |
|
Number of tumors |
36 |
|
Tumors <1.0cm |
15 |
|
Tumors <1.0cm |
21 |
|
Limb/paw |
21 |
|
Trunk |
10 |
|
Head |
3 |
|
Genitalia |
2 |
Table 1: Signalment and Tumor Profile.
Location of the tumors showed a preponderance of locations. However, most tumors were on the caudal half of the dog’s body and on the limbs or paws (n=21, 58.3%). All tumors were less than 1.8 cm maximum diameter due to current recommended limitations of the therapeutic handpiece by the manufacturer, however 41.6% (n=15) of the treated tumors were less than 1.0 cm maximum diameter in their largest dimension and 58.3% (n=21) of the treated tumors were greater than 1.0 cm maximum diameter in their largest dimension, respectively (Table 1).
3.2. Treatment and Outcome
Treated dogs had a 100% response rate, with 94% (n=34 tumors) achieving clinical remission (CR). Progression was noted in 8 treatment tumors (22% of tumors), two of which had only achieved a partial remission (PR). Overall recurrence rate (RR) was 21%. Recurrence Rate (RR) in tumors achieving clinical remission was only 17%. (Table 2)
|
Response rate |
100% |
|
Clinical remission |
34(96%) |
|
Partial remission |
2(4%) |
|
Out of remission |
6 |
|
Progression |
2 |
|
Recurrence rate |
21%(all)&17%(w/o PR dogs) |
Table 2: Basic Statistics.
Time to complete response took on average 20 days. Toxicities in treated dogs were limited to minor dermal thermal burns/wounds, necrosis of tumor, scabs, swelling of tumor to 48 hours, and rare short-lived pain (<24 hours). All toxicities were resolved by 14 days. Antibiotic use was limited to dogs (5) who had self-trauma induced infections. All treated sites healed without long term sequelae (Table 3).
|
Toxicites |
Minor dermal burns, swelling, necrosis of tumor, scabs, short lived pain |
|
Duration of toxicites |
<14days |
Table 3: Toxicity Data.
At the end of the study, 24 dogs (80%), were still alive, and 6 (20%) had died because of MCT-unrelated causes. Mean progression free time (PFT) was 552 days and median progression free time was not reached. All tumors were censored because patients were still in clinical remission or due to death of the dog from other diseases. Kaplan Meier remission probability statistics and curve are reported (Table 4 and Figure 2).
|
Mean Progression Free Time |
552 days |
|
Confidence ratio for PFT |
472.433 to 631.635 |
|
Median Progression Free time |
Not reached |
|
Censored tumors |
29 |
|
Median Survival Time |
Not Reached |
|
Death |
6 (Hemangiosarcoma, pericardial effusion, and unknown) |
Table 4: Kaplan Meier Statistics.
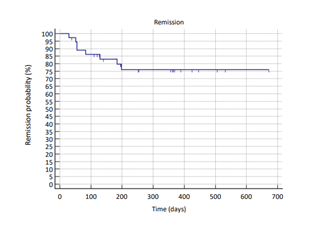
Figure 2: Kaplan Meyer remission probability curve (blue line). Hash marks indicate date of censoring.
4. Discussion
The purpose of this retrospective analysis was to evaluate the efficacy of PTA via the use of GSNs and laser light (808nm) in the local treatment of cutaneous and subcutaneous low grade MCT in canines. Even though current successful treatments exist for canine MCT, their prevalence, location being cutaneous and subcutaneous, and general size makes them candidates for PTA when recommendations for surgery, RT or ECT, etc. are either declined or not desired for various reasons. The gold standard therapy for low grade (two grade system-Kiupel) MCT is considered surgical removal or an equivalent local therapy due to the low risk of metastasis of these tumors [37]. The main finding of this retrospective analysis was that PTA provided effective local control of canine low grade MCT with minimal self-resolving toxicities. The responses matched and, in some cases, exceeded local control reported in narrowly (<0.3cm margins) or incompletely surgically resected tumors, or tumors treated with radiation or electrochemotherapy [32, 33, 47].In our analysis, all PTA treated tumors responded (RR=100%) with all but two tumors achieving a clinical remission (94% CR). A majority of PTA treated MCT had extended progression free times, with 78% of tumors (28) being in clinical remission at time of last follow up. Mean progression free time was 552 days. Interestingly, two of the eight tumors that had progression had only achieved a PR and when PFT was evaluated with just the tumors achieving CR, the recurrence rate was only 17%. Overall recurrence rate in this study (21%) was in the lower end of the recurrence rate reported for incompletely surgically resected MCT or tumors resected with <0.3cm margins (12-63%), suggesting that PTA could be equivalent to surgical resection with incomplete margins or narrow margins [31,37,41,43-46] Response Rate (RR) and CR rates were higher than previously reported responses for radiation therapy (RR= 41- 88% and CR= 34-59%) and electro chemotherapy (RR-80-100% and CR = 70%) [47, 49-52]. Caution should be taken when comparing to radiation and electrochemotherapy as the studies on these therapies included high grade and larger tumors. Whereas, this analysis included only patients with small tumors (<1.8cm diameter) and no obvious high-grade tumors. Thus, apparent differences in response between these local therapies and PTA could be due to grade and size of the MCT in the studies [47, 49-52]. When comparing PTA recurrence rates to those reported for electro chemotherapy and radiation therapy (23-50%), PTA was more effective in establishing long term disease control [47, 49, 50, 52]. Once again the differences in these studies populations and their inclusion criteria may hamper comparisons to local therapies that are not surgical. Because of the paucity of histopathologic evaluation compared to cytologic evaluation in the patient data analyzed in this study, the authors did not distinguish between cutaneous and subcutaneous tumors during statistical analysis. However, in previous studies subcutaneous MCT have lower chances of metastasis and similar rates of local recurrence after incomplete or narrowly excised tumors [37, 43, 55]. Therefore, it is the author’s opinion that the data is not weakened by not distinguishing between cutaneous and subcutaneous tumors. Still, this should be taken into consideration in future comparisons between surgical treatment and PTA, especially as it is suggested that it is easier to achieve surgical margins with cutaneous tumors [32, 40, 44]. There are limitations to this retrospective analysis. Primarily, all tumors were less than 1.8cm in their maximum dimension. This limitation was based on the predictive modeling used and previous pilot data, and required to achieve adequate irradiance for transcutaneous laser PTA, efficacy for larger MCT, particularly in the dimension of tissue depth, cannot be assumed.
Location of MCTs have been embroiled in long controversy involving recurrence rates and disease control with some suggesting that certain locations have shorter PFT. Most of the controversy is likely related the ability in achieving adequate surgical resection of the tumor. The majority (72% [n=26]) of treated dogs in this study had tumors in the locations previously suggested to have lower disease control with surgery and 21 tumors (58.3%) were in locations reported more likely to have incisional complications than tumors in other locations [53]. In the current analysis, all toxicities noted with PTA treatment were minor and self-resolving, including minor dermal burns, necrotic tumor tissue, and some minimal pain. All toxicities resolved by two weeks and none of the PTA sites required corrective surgery or long-term bandaging. Medical therapy for pain was rare and usually stopped within 24 hours of the laser treatment. Although this is not a prospective study with matched control group, due to the wealth of historical data on MCT after surgical resection and other treatments comparisons to previously published data were still possible and described above. In the future it would be advantageous to ensure all tumors have a histopathologic analysis to allow further determination of relationships between grade, cutaneous, fixed, invasive, and subcutaneous MCTs. Future studies should include repeated PTA therapy with and without GSN infusions, to assess whether those tumors with only a partial response could be converted into full clinical remissions. It would also be advantageous to assess if repeating PTA after recurrence could provide a second response and/or clinical remission. It will be key in such studies to assess the ability of tumors to retain GSN particles long term and/or for tumor tissue to accumulate GSN after repeat infusions and respond to additional photothermal ablation. Answers to these questions could provide evidence for the need for future infusions of GSN and/or what impact a second infusion might have on the patients and tumors.
5. Conclusions
In conclusion results of this study suggest that using gold-coated silicone nanoparticles and exposure to near infrared light (808 nm) for photothermal ablation provides an effective local therapy of low-grade mast cell tumors. Median progression free time and survival was not reached in the treated patients, suggesting that long term tumor control is possible with PTA that potentially equals surgery when margins are narrow (<0.3cm) or incomplete. PTA appears to have better and more durable MCT responses than either radiation therapy or electrochemotherapy when used as sole therapies. Toxicities in this study were minor and resolved without surgical intervention. Future studies should include more cases of different grades of MCT, second PTA for those not fully achieving clinical remission or recurrent tumors, assessment of long term GSN retention in tumors, and evaluation of other cutaneous, subcutaneous, or easily illuminated tumor types.
Funding
Funding for treatment was provided by Companion Animal Health and was offered as a minimal stipend paid towards the treatment of dogs included as study participants.
General
The authors gratefully acknowledge the support of the pet owners and practice staff, as well as veterinary colleagues and they acknowledge the impact of cited references on the analysis of this work.
Competing interests
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Authors LM and LDT are currently employed by Companion Animal Health (LiteCure) and were involved in the article preparation.
Consent for publication
All authors give consent for publication
Availability of data and materials
All data is available for evaluation upon request
Acknowledgment
All authors provide their consent for publication. Data and materials from the study are available upon request from the corresponding author.
References
- Bertrand N, Wu J, Xu X, et al. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 66 (2014): 2-25.
- Zhang L, Gu FX, Chan JM, et al. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clinical pharmacology & Therapeutics 83 (2008): 5.
- Jain S, Hirst D, Sullivan M. Gold Nanoparticles as Novel Agents for Cancer Therapy. The British Journal of Radiology 85 (2012): 101-113.
- Schwartz JA, Price RE, Gill-Sharp KL, et al. Selective nanoparticle-directed ablation of the canine prostate. Lasers Surg. Med 43 (2011): 213-220.
- Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 100 (2003): 13549-13554.
- Abadeer NS, Murphy CJ. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem 120 (2016): 4691-4716.
- Loo C, Lin A, Hirsch L, et al. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat 3 (2004): 33-40.
- Rastinehead AR, Anastos H, Wajswol E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. PNAS 116 (2019): 18590-18596.
- Stern JM, Solomonov VV, Sazykina E, et al. Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. International journal of toxicology 35 (2016): 38-46.
- Stern JM, Stanfield J, Kabbani W, et al. Selective Prostate Cancer Thermal Ablation With Laser Activated Gold Nanoshells. J of Urology 179 (2008): 748-753.
- Kydd J, Rahul J, Velpurisiva P, et al. Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems. Pharmaceutics 9 (2017): 46.
- Chamseddine I, Kokkolaras M. Nanoparticle optimization for enhanced targeted anti-cancer drug delivery. J Biomech Eng 140 (2017).
- Fred Hutchison Cancer Institute.
- O’Neal DP, Hirsch LR, Halas NJ, et al. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett 209 (2004): 171-176.
- Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: Impending clinical impact. Acc Chem Res (2008): 41, 1842-1851.
- Huang X, El-Sayed IH, Qian W, et al. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc 128 (2006): 2115-2120.
- Guo L, Niu G, Zheng X, et al. Single Near-Infrared Emissive Polymer Nanoparticles as Versatile Phototheranostics. Adv Sci (Weinh) 10 (2017).
- Rancoule C,Magne N, Vallard A, et al. Nanoparticles in radiation oncology: From bench-side to bedside. Cancer Letters 375 (2016): 256-262.
- Allegra A, Penna G, Alonci A, et al. Nanoparticles in Oncology: The New Theragnostic Molecules. Anti-Cancer Agents in Medicinal Chemistry 11 (2011): 669-686.
- Ferrari Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5 (2005): 161-171.
- Grobmyer SR, Iwakuma N, Sharma, et al. What is cancer Nanotechnology? In Cancer Nanotechnology, Methods in Molecular Biology. Eds. Stephen Grobmyer and Brij Moudgil No. 624. Humana Press 2010: Springer Science+ Business LLC (2010): 1-9.
- ASTM International. E 2456-06 Terminology for nanotechnology. West Conshohocken, PA: ASTM International (2006).
- Vertegel AA, Siegel RW, Dordick JS. Silica nanoparticle size influences the structure and enzymatic activity of adsorbed lysozyme. Langmuir 20 (2004): 6800-6807.
- Wang B, Zhai Y, Shi J, et al. Simultaneously overcome tumor vascular endothelium and extracellular matrix barriers via a non-destructive size-controlled nanomedicine. J Control Release 268 (2017): 225-236.
- Kaur P, Aliru ML, Chadha AS, et al. Hyperthermia Using Nanoparticles - Promises and Pitfalls. Int J Hyperthermia 32 (2016): 76-88.
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 41 (2001): 189-207.
- Magaña IB, Yendluri RB, Adhikari P, et al. Suppression of the reticuloendothelial system using λ-carrageenan to prolong the circulation of gold nanoparticles. Ther Deliv 6 (2015): 777-83.
- Tang F, Zhang Y, Zhang J, et al. Assessment of the efficacy of laser hyperthermia and nanoparticle-enhanced therapies by heat shock protein analysis. AIP Advances 4 (2014): 031334.
- Gad SC, Sharp KL, Montgomery C, et al. Evaluation of the Toxicity of Intravenous Delivery of Auroshell Particles (Gold-Silica Nanoshells). Intl J of Toxicology 31 (2012): 584-594.
- Schwartz JA, Shetty AM, Price RE, et al. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model.Cancer Res 69 (2009): 1659-1667.
- London C, Thamm D. Mast Cell Tumors. 5th ed. St Louis, MO: Saunders Elsevier (2013).
- Garrett L. Canine mast cell tumors: diagnosis, treatment, and prognosis. Vet Med (Auckl) 5 (2014): 49-58.
- Withrow. MacEwen’s Small Animal Clinical Oncology. Editors David Vail, Douglas Thamm, Julius Liptak (2019): section 21.
- Kiupel M, CamusDiagnosis and Prognosis of Canine Cutaneous Mast Cell Tumors. Vet Clin of NA: Small Animal Practice 49 (2019): 819-836.
- Mullins M, Dernell W, Withrow S, et al. Evaluation of prognostic factors associated with outcome in dogs with multiple cutaneous mast cell tumors treated with surgery with and without adjuvant treatment: 54 cases (1998-2004). J Am Vet Med Assoc 228(2006): 91-95.
- Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol 21 (1984): 469-474.
- Kiupel M, Webster JD, Bailey KL, et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol 48 (2011): 147-155.
- Bacon NJ, Dernell WS, Ehrhart N, et al. Evaluation of primary re-excision after recent inadequate resection of soft tissue sarcomas in dogs: 41 cases (1999-2004) J Am Vet Med Assoc 230 (2007): 548-554.
- Simpson AM, Ludwig LL, Newman SJ. Evaluation of surgical margins required for complete excision of cutaneous mast cell tumors in dogs. J Am Vet Med Assoc 224 (2004): 263-240.
- Selmic L and Ruple A. A systematic review of surgical margins utilized for removal of cutaneous mast cell tumors in dogs. BMC Veterinary Research 16 (2020): 5.
- Smiech A, Slaska B, Lopuszynski W, et al. Epidemiological assessment of the risk of canine mast cell tumours based on the Kiupel two-grade malignancy classification. Acta Vet Scand 60 (2018):70.
- Cahalane AK, Payne S, Barber LG, et al. Prognostic factors for survival of dogs with inguinal and perineal mast cell tumors treated surgically with or without adjunctive treatment: 68 cases (1994-2002). J Am Vet Med Assoc 225 (2004): 401-408.
- Thompson J, Pearl DL, Yager JA, et al. Canine Subcutaneous Mast Cell Tumor: Characterization and Prognostic Indices. Veterinary Pathology 48 (2011): 156-168.
- Milovancev M, Townsend KL, Tuohy JL, et al. Long-term outcomes of dogs undergoing surgical resection of mast cell tumors and soft tissue sarcomas: A prospective 2-year-long study. The American College of Veterinary Surgeons, Veterinary Surgery 49 (2019): 96-105.
- Weisse C, Shofer FS, Soremno K. Recurrence rates and sites for grade II canine cutaneous mast cell tumors following complete surgical excision. J Am Anim Hosp Assoc 38 (2002): 71-73.
- Michels GM, Knapp DW, DeNicola DB, et alPrognosis following surgical excision of canine cutanous mast cell tumors with histopathologically tumor-free versus nontumor-free margins: a retrospective study of 31 cases. J Am Anim Hosp Assoc 38 (2002): 458-466.
- Cemazar M, Tamzali Y, Sersa G. Electrochemotherapy in veterinary oncology. J Vet Intern Med 22 (2008): 826-831.
- Kry KL, Boston SE. Additional local therapy with primary re-excision or radiation therapy improves survival and local control after incomplete or close surgical excision of mast cell tumors in dogs. Vet Surg 43 (2014): 182-189.
- Dobson J, Cohen S, Gould S. Treatment of canine mast cell tumours with prednisolone and radiotherapy. Vet Comp Oncol 2 (2004): 132-141.
- Lejeune A, Skorupski K, Frazier S , et al. Aggressive local therapy combined with systemic chemotherapy provides long-term control in grade II stage 2 canine mast cell tumour: 21 cases (1999-2012). Vet Comp Oncol 13 (2015): 267-280.
- Carlsten KS, London CA, Haney S, et. al. Multicenter Prospective Trial of Hypofractionated Radiation Treatment, Toceranib, and Prednisone for Measurable Canine Mast Cell Tumors. J Vet Intern Med 26 (2012): 135-141.
- Kodre V, Cemazar M, Pecar J, et al., Electrochemotherapy Compared to Surgery for Treatment of Canine Mast Cell Tumours. In Vivo 23 (2009): 55-62.
- Iodence AE, Wallace ML, Grimes JA, et al. Dogs undergoing surgical excision of mast cell tumors are not at increased risk of incisional complications, Journal of the American Veterinary Medical Association 260 (2022): S88-S95.
- Nguyen SM, Thamm DH, Vail DM,et al. Response evaluation criteria for solid tumours in dogs Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol 13 (2015): 176-183.
- Newmana SJ, Mrkonjich L, Walker KK & Rohrbach BW. Canine subcutaneous mast cell tumour: diagnosis and prognosis. Journal of Comparative Pathology 136 (2007): 231-239.
