Tumour-Associated Tissue Factor (TF)-mRNA Is A Precursor for Rapid TF-Microvesicle Release and A Potential Predictive Marker for the Risk of Pulmonary Embolism (PE) in Gastrointestinal Cancer Patients
Article Information
Camille Ettelaie1*, Naima E Benelhaj2, Sophie Featherby1, Farzana Haque2, Miriam J Johnson2, Justin Cooke3, Anthony Maraveyas2
1Biomedical Section, University of Hull, Cottingham Road, Hull, HU6 7RX, UK
2Hull York Medical School, University of Hull, Cottingham Road, Hull, HU6 7RX, UK
3Department of Pathology, Hull Royal Infirmary, Hull University Teaching Hospital NHS Trust, Anlaby Road, Hull, HU3 2JZ, UK
*Corresponding author: Camille Ettelaie, Biomedical Section, University of Hull, Cottingham Road, Hull, HU6 7RX, UK
Received: 09 May 2022; Accepted: 17 May 2022; Published: 21 May 2022
Citation: Camille Ettelaie, Naima E. Benelhaj, Sophie Featherby, Farzana Haque, Miriam J Johnson, Justin Cooke, Anthony Maraveyas. Tumour-associated tissue factor (TF)-mRNA is a precursor for rapid TF-microvesicle release and a potential predictive marker for the risk of pulmonary embolism (PE) in gastrointestinal cancer patients. Journal of Biotechnology and Biomedicine 5 (2022): 78-93.
View / Download Pdf Share at FacebookAbstract
Predicting which cancer patients are at risk of thrombosis remains a key challenge to effective thromboprophylaxis. This study was based on the hypothesis that the rapid release of TF-containing MV occurs in cancer cells that possess high levels of TF mRNA, permitting the transient but amplified TF-protein production in response to cellular activation. To gather preliminary clinical evidence for this hypothesis the correlation between the levels of tumour-associated TF mRNA and incidence of Pulmonary Embolism (PE) in Gastrointestinal cancer patients (GI) was assessed using stringently-selected patient cohorts. Furthermore, the rapid TF-MV release was assessed in three cell lines with high TF mRNA in which protein-translation or mRNA-transcription were inhibited separately. On applying the exclusion criteria, the study accrued 9 clinical samples with incidental PE (colonic n=5; gastroesophageal n=4) which were type, gender and stage of cancer matched one-to-one with patients without PE (9+9 samples). Total-RNA was extracted from the samples using a FFPE-RNA extraction kit and TF mRNA was quantified using a quantitative real-time PCR procedure along with a standard curve prepared using in vitro-transcribed TF mRNA. Relative amounts of PAR2 mRNA were also determined. Analysis of absolute amounts of tumour-associated TF mRNA showed significant increase in patients who developed PE (mean=26.931±15.371 pg/100ng-total RNA; median=5.340 pg/100ng-total RNA; range=0.4-131.43 pg/100ng-total RNA) compared to those who didn’t (mean=0.098±0.023 pg/100ng-total RNA; median=0.110 pg/100ng-total RNA; range=0-0.19 pg/100ng-total RNA). Receiver Operating Characteristic (ROC) analysis returned an area under the curve of 1. In contrast, no significant difference in PAR2 mRNA was recorded. To provide an explanation for these findings, inhibition of protein-translation using cycloheximide prevented the incorporation of TF but not the MV release. However, blocking of
Keywords
Cancer-associated thrombosis; Gastrointestinal cancer; Messenger RNA; Pulmonary embolism; Tissue factor; PAR2
Cancer-associated thrombosis articles Cancer-associated thrombosis Research articles Cancer-associated thrombosis review articles Cancer-associated thrombosis PubMed articles Cancer-associated thrombosis PubMed Central articles Cancer-associated thrombosis 2023 articles Cancer-associated thrombosis 2024 articles Cancer-associated thrombosis Scopus articles Cancer-associated thrombosis impact factor journals Cancer-associated thrombosis Scopus journals Cancer-associated thrombosis PubMed journals Cancer-associated thrombosis medical journals Cancer-associated thrombosis free journals Cancer-associated thrombosis best journals Cancer-associated thrombosis top journals Cancer-associated thrombosis free medical journals Cancer-associated thrombosis famous journals Cancer-associated thrombosis Google Scholar indexed journals Gastrointestinal cancer articles Gastrointestinal cancer Research articles Gastrointestinal cancer review articles Gastrointestinal cancer PubMed articles Gastrointestinal cancer PubMed Central articles Gastrointestinal cancer 2023 articles Gastrointestinal cancer 2024 articles Gastrointestinal cancer Scopus articles Gastrointestinal cancer impact factor journals Gastrointestinal cancer Scopus journals Gastrointestinal cancer PubMed journals Gastrointestinal cancer medical journals Gastrointestinal cancer free journals Gastrointestinal cancer best journals Gastrointestinal cancer top journals Gastrointestinal cancer free medical journals Gastrointestinal cancer famous journals Gastrointestinal cancer Google Scholar indexed journals Messenger RNA articles Messenger RNA Research articles Messenger RNA review articles Messenger RNA PubMed articles Messenger RNA PubMed Central articles Messenger RNA 2023 articles Messenger RNA 2024 articles Messenger RNA Scopus articles Messenger RNA impact factor journals Messenger RNA Scopus journals Messenger RNA PubMed journals Messenger RNA medical journals Messenger RNA free journals Messenger RNA best journals Messenger RNA top journals Messenger RNA free medical journals Messenger RNA famous journals Messenger RNA Google Scholar indexed journals Pulmonary embolism articles Pulmonary embolism Research articles Pulmonary embolism review articles Pulmonary embolism PubMed articles Pulmonary embolism PubMed Central articles Pulmonary embolism 2023 articles Pulmonary embolism 2024 articles Pulmonary embolism Scopus articles Pulmonary embolism impact factor journals Pulmonary embolism Scopus journals Pulmonary embolism PubMed journals Pulmonary embolism medical journals Pulmonary embolism free journals Pulmonary embolism best journals Pulmonary embolism top journals Pulmonary embolism free medical journals Pulmonary embolism famous journals Pulmonary embolism Google Scholar indexed journals Tissue factor articles Tissue factor Research articles Tissue factor review articles Tissue factor PubMed articles Tissue factor PubMed Central articles Tissue factor 2023 articles Tissue factor 2024 articles Tissue factor Scopus articles Tissue factor impact factor journals Tissue factor Scopus journals Tissue factor PubMed journals Tissue factor medical journals Tissue factor free journals Tissue factor best journals Tissue factor top journals Tissue factor free medical journals Tissue factor famous journals Tissue factor Google Scholar indexed journals PAR2 articles PAR2 Research articles PAR2 review articles PAR2 PubMed articles PAR2 PubMed Central articles PAR2 2023 articles PAR2 2024 articles PAR2 Scopus articles PAR2 impact factor journals PAR2 Scopus journals PAR2 PubMed journals PAR2 medical journals PAR2 free journals PAR2 best journals PAR2 top journals PAR2 free medical journals PAR2 famous journals PAR2 Google Scholar indexed journals Microvesicles articles Microvesicles Research articles Microvesicles review articles Microvesicles PubMed articles Microvesicles PubMed Central articles Microvesicles 2023 articles Microvesicles 2024 articles Microvesicles Scopus articles Microvesicles impact factor journals Microvesicles Scopus journals Microvesicles PubMed journals Microvesicles medical journals Microvesicles free journals Microvesicles best journals Microvesicles top journals Microvesicles free medical journals Microvesicles famous journals Microvesicles Google Scholar indexed journals Thrombosis articles Thrombosis Research articles Thrombosis review articles Thrombosis PubMed articles Thrombosis PubMed Central articles Thrombosis 2023 articles Thrombosis 2024 articles Thrombosis Scopus articles Thrombosis impact factor journals Thrombosis Scopus journals Thrombosis PubMed journals Thrombosis medical journals Thrombosis free journals Thrombosis best journals Thrombosis top journals Thrombosis free medical journals Thrombosis famous journals Thrombosis Google Scholar indexed journals Cancer articles Cancer Research articles Cancer review articles Cancer PubMed articles Cancer PubMed Central articles Cancer 2023 articles Cancer 2024 articles Cancer Scopus articles Cancer impact factor journals Cancer Scopus journals Cancer PubMed journals Cancer medical journals Cancer free journals Cancer best journals Cancer top journals Cancer free medical journals Cancer famous journals Cancer Google Scholar indexed journals Diagnostics articles Diagnostics Research articles Diagnostics review articles Diagnostics PubMed articles Diagnostics PubMed Central articles Diagnostics 2023 articles Diagnostics 2024 articles Diagnostics Scopus articles Diagnostics impact factor journals Diagnostics Scopus journals Diagnostics PubMed journals Diagnostics medical journals Diagnostics free journals Diagnostics best journals Diagnostics top journals Diagnostics free medical journals Diagnostics famous journals Diagnostics Google Scholar indexed journals
Article Details
Abbreviations:
TF: Tissue factor; fVII/X: Factor VII/X; fVIIa/Xa: Activated factor VII/X; PAR2: Protease activated receptor 2; MV: Microvesicles (cell derived); PE/IPE : Pulmonary embolism/Incidental pulmonary embolism; VTE: Venous thromboembolism; DVT: Deep vein thrombosis; CAT: Cancer associated thrombosis; GI: Gastrointestinal
1. Introduction
A key challenge in management of Cancer Associated Thrombosis (CAT) is the effective risk assessment to enable clinically relevant prophylaxis in order to reduce mortality and morbidity from CAT. Currently available Clinical Prediction Rules (CPRs) such as the Khorana score rely heavily on clinical factors such as tumour type, cancer stage, patient habitus and the effects of cancer on conventional laboratory blood parameters [1-3]. A few circulating indicators for thrombosis such as D-dimers or P-selectin have been incorporated into these tools, yet their efficacy remains limited. Previous clinico-pathological studies suggest that tumour-associated Tissue Factor (TF) may be a predictor of poor prognosis in some cancers [4-7]. Particularly, TF expression correlated with the histological grade of malignancy and is associated with increased tumour dissemination and angiogenesis in several types of cancer [4, 8-9]. TF, together with factor VIIa (fVIIa) acts as the principal initiator of blood coagulation. Both TF-fVIIa complex and the ternary TF-fVIIa-fXa complex are capable of activating Protease Activated Receptor 2 (PAR2) [10] triggering Microvesicle (MV) release [11]. Upregulation of PAR2 has also been demonstrated during cancers [12-14]. Gastrointestinal (GI) cancers (pancreas, stomach, liver, colon, rectum) are reported to be among cancers with the highest incidence of Deep Vein Thrombosis (DVT) or Pulmonary Embolism (PE) [15]. Therefore, coagulation proteins including TF may be useful markers for the risk of thrombosis in GI cancers. Analyses of TF in tumours have mainly included the measurement of TF antigen or activity on the cell surface [4-9]. Additionally, the association between the risk of thrombosis in cancer patients and the release of procoagulant tumour-microvesicles into the blood circulation has been reported [16-21]. Elevated levels of these microvesicles, harbouring TF and phosphatidylserine are often associated with the hypercoagulable state [16-18]. Therefore, patients with high circulating TF levels have significantly increased risk of recurrent VTE, even while on anticoagulation [18-21] and at least one study reported TF as a baseline predictor for VTE risk [21]. However, despite these associations, there is no clear quantitative interrelation between the concentration of circulating procoagulant microvesicles and the incidence of thromboembolism [18, 19]. Moreover, data from studies examining the correlation between the risk of thrombosis and the level of total-cell and cell-surface TF antigen, or the cell-surface TF activity have been heterogeneous [16-18].
Previously we examined the potential of 17 cell lines (including gastrointestinal cancers) to release TF-bearing microvesicles following activation of PAR2 [22]. The level of microvesicle-associated TF during the short release burst strongly correlated with both the TF mRNA and PAR2 mRNA expression levels but not TF-protein. The activation of PAR2 has been shown to promote the phosphorylation of p90RSK, mediated through the rapid activation of the MAPK pathway [23], closely followed by the release of TF-MV [22]. We therefore hypothesised that analysis of TF mRNA and PAR2 mRNA levels may provide valuable information to determine the risk of thrombosis. This study aimed to examine the clinical significance of TF mRNA and PAR2 mRNA expression in tumour tissue from GI cancer patients, with or without incidental PE (IPE). Additionally, using an in vitro model, we examined the hypothesis that the accumulated TF mRNA within the cancer cell lines acts as a precursor for rapid TF protein production and release within MV.
2. Material and Methods
2.1. Patients
A previously described cohort of 234 ambulatory adult cancer patients that attended the acute service, with a first diagnosis of an incidental PE were screened for this pilot study [24] and for these, we have collected comprehensive epidemiological and biological data on the cohort from as far back as 2010. The selection criteria included: age of 18 years or over, tumour type (gastroesophageal, hepatopancreaticobiliary and colorectal adenocarcinoma), presence of proximal unilateral or bilateral PE (main pulmonary artery, left and right main pulmonary arteries, anterior trunk, right and left interlobar arteries, left upper lobe trunk, right middle lobe artery, and the right and left lower lobe arteries), the availability of a pathological sample of a resected primary cancer, M1 stage at the diagnosis of the IPE and the absence of other identifiable known risk factors for VTE (e.g. Central Venous Access Devices (CVAD), previous VTE, recent surgery immobilisation etc.). Therefore, any subjects with VTE that were perceived to arise from
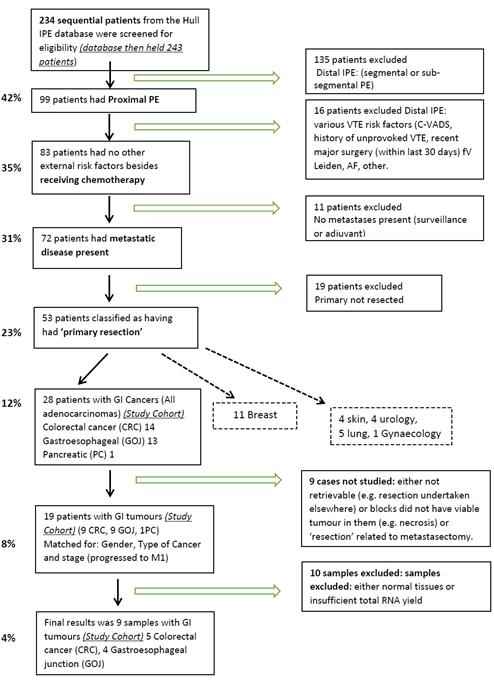
Figure 1: Flow chart of the patient selection procedure.
causes independent of the cancer were omitted from the study. Once the target cohort was obtained (Figure 1), matching cases from the relevant Multidisciplinary Team Meetings’ (MDT-Somerset) databases were obtained. Patients were matched on a one-to-one basis with cases that had no PE or VTE history by the chief investigator (AM) and link anonymised. Matching parameters were gender, age ±5 years, type of cancer and M1 stage with a history of previous treatment with at least one course of chemotherapy. Ethical approval, IRAS project ID: 185389 REC reference: 16/NW/0378, was obtained prior to the study. Selected sets of Formaldehyde-Fixed Paraffin Embedded (FFPE) tissue samples from both groups were reviewed by a pathologist (JC) for the existence of adequate cancerous tissue and link anonymised blocks of tissue were provided to the investigating laboratory.
2.2. Isolation of total RNA from FFPE tissue and RT-PCR
FFPE tissue sections were cut (10-15 μm thick) from the same block and immediately placed in a 1.5 ml collection tube. Histo-Clear II (1ml/tube; National Diagnostics, Hessel, UK) was pipetted into the tube, vortexed for 10s and incubated for 10 min with shaking. The tubes were then centrifuged for 2 min, supernatant discarded and the procedure repeated two more times. The tissue samples were then incubated with absolute ethanol (1 ml) twice and then incubated with progressive-stepwise dilutions of ethanol in water to 70% (v/v). After each incubation, the pellet was centrifuge and the supernatant discarded. The rehydrated pellet was used immediately for extraction of total RNA. Total RNA was isolated from the deparaffinised/rehydrated tissue sections using the Qiagen RNeasy FFPE Kit (Qiagen, Manchester, UK) according to the manufacturer’s instructions. The absolute amount of TF mRNA and the relative amount of PAR2 mRNA were determined using QuantiTect primer sets to detect either TF or PAR2, together with β-actin (Qiagen). The reactions were carried out at an annealing temperature of 60°C for 1 min using the GoTaq® 1-Step RT-qPCR System (Promega Corporation Ltd, Southampton, UK) on an iCycler thermal-cycler (Bio-Rad, Hemel Hempstead, UK) for 40 cycles. Following amplification, the Ct values were normalised against the amount of β-actin and the absolute amounts of TF mRNA were determined using a standard curve prepared using in vitro-transcribed TF mRNA, previously prepared according to published procedures [25]. An attempt was carried out to extract proteins from the FFPE tissue samples, using the Qproteome FFPE Tissue Kit (Qiagen). However, the procedure did not produce usable protein samples because the FFPE extraction reagents were not compatible with either the Quantikine TF-ELISA kit (R&D Systems, Abingdon, UK) or SDS-PAGE procedure.
2.3. Cell culture, Microvesicles (MV) and quantification
Cell lines originating from gastrointestinal cancers were obtained from ATCC (Teddington, UK) and selected because of high content of TF mRNA as well as amplified abilities to release MV-associated TF as determined previously [22]. ASPC-1 pancreas adenocarcinoma cell line was cultured in RPMI-1640; CaCo-2 colorectal adenocarcinoma cells were cultured in EMEM and LoVo grade IV Dukes C colorectal adenocarcinoma cells were cultured in Ham’s F-12 K medium. All media were obtained from Lonza (Cambridge, UK) and contained foetal calf serum 10 % (v/v; Source Bioscience plc, Nottingham, UK). Cells (2 × 105) were seeded out in 12-well plates and permitted to adhere and then washed with PBS and pre-adapted to respective serum-free medium. Sets of cells were supplemented with cycloheximide (10 nM; Sigma Chemical Company Ltd., Poole, UK) to prevent protein translation, or with excess amounts of actinomycin D (1 µM; Santa Cruz Biotechnology/Insight Biotechnology, Wembley, UK) to block mRNA transcription, or the vehicle DMSO (0.02% w/v) and incubated for 1 h. To induce microvesicle release, the cells were stimulated with PAR2-activating peptide (PAR2-AP); SLIGKV; (20 μM) (Sigma). Conditioned media were collected after 30 min and the microvesicle densities were quantified using the Zymuphen MP Assay kit (Hyphen BioMed/ Quadratech Ltd, Epsom, UK). The identities of the MV preparations were confirmed previously [26].
2.4. Quantification of microvesicle-associated TF antigen and activity
The TF antigen associated with the microvesicles was measured using the Quantikine TF-ELISA kit (R&D Systems) according to the manufacturers' instructions [27]. Microvesicle TF-fVIIa activities were measured by modification of previously described procedures [27]. To measure TF activity, microvesicle samples were captured onto the 96-well plate using the Zymuphen system. The samples were washed with 50 mM Tris-buffered saline pH 7.2 (300 µl) and the thrombin generation potential determined as previously described using a two-stage amidolytic assay based on the liberation of a chromogenic group from the substrate by the generated thrombin [22,25] and detailed before [28]. Briefly, samples of the isolated MV (20 μl) were incubated with 25 mM CaCl2 (20 μl), 5 mg/ml barium-adsorbed plasma solution (20 μl) and 0.05 M Tris–HCl buffer pH 7.2 (40 μl) in a 96-well plate at 37°C. After 20 min, the generation of thrombin was quenched by the addition of substrate buffer (0.05M Tris-HCl buffer pH 7.2, 25 mM EDTA) (100 μl) containing 0.1mM chromogenic CS-01(81) substrate (Hyphen BioMed). The samples were then incubated for up to 30 min to develop the colour, the reactions were then stopped using 2% (v/v) acetic acid (50 µl) and the absorptions measured immediately at 410 nm. The TF activities were determined by comparison to a range of recombinant TF (Innovin recombinant human tissue factor reagent; Dade Behring, Liederbach, Germany) according to the TF activity (1 U/ml=0.13 ng/ml).
2.5. Statistical plan and analysis
The plan of this pilot study was based on the speculated number of cases from the IPE database that would fulfil the criteria. A maximum sample of 50 cases (total of 100 matched cases) was assumed. Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS Inc. Chicago, USA). Descriptive analysis of samples was carried out, including Kolmogorov-Smirnov analysis to determine the distribution of the data and Mann-Whitney test to ascertain the significance of the difference between the two groups. The sensitivity and specificity of TF mRNA concentrations within the tumour tissue samples were determined with the receiver operating characteristic (ROC) curve analysis. Significance of the differences between the MV densities and the TF contents were determined using one-way ANOVA (analysis of variance) and Tukey’s honesty significance test or where appropriate, by paired t-test and p values of equal or less than 0.05 were deemed to be significant.
3. Results
TF mRNA expression in 18 human gastrointestinal cancers (9 IPE including and 9 matched samples with no PE), were measured using quantitative RT-PCR. A standard curve was prepared using a range of in vitro-transcribed TF mRNA and the Ct values determined. The presence of cancer cells was examined in half of the FFPE samples, which contained comparable but not identical number of cells. The Ct data were normalised against β-actin mRNA in each sample and the absolute amount of TF mRNA pg/100 ng-total RNA was then determined and plotted for each matched pair (Figure 2). Descriptive analysis of the data indicated skewed distribution of the data but with significant differences (Mean; IPE=26.931±15.371 pg/100 ng-total RNA vs. no-PE=0.098±0.023 pg/100 ng-total RNA, Median; IPE=5.340 pg/100 ng-total RNA vs. no-PE=0.110 pg/100 ng-total RNA). The Mann-Whitney test was used to demonstrate the difference of the TF mRNA measurements in the two groups with significance (p <0.001 vs. no-PE sample). The average Ct value of PAR-2 mRNA, after normalisation against β-actin mRNA was 36.85±1.88 for tissue samples from patients with IPE, compared to 36.25±2.87 for those without PE. Since these figures were almost identical, no further evaluation of the data was carried out. In order to explore and confirm the source of the released TF protein, three cells lines with elevated levels of TF mRNA and the potential to release high densities of TF-MV were employed. The absolute values of TF mRNA content of the three cell lines were previously quantified using a similar RT-qPCR procedure used here and shown to be high in these cell lines, compared to a set of 17 various cells lines. The cell lines were incubated separately with cycloheximide (10 nM) to prevent protein translation, actinomycin D (1 µM) to prevent mRNA transcription or the vehicle DMSO (0.02% w/v). Activation of PAR2 using the SLIGKV peptide resulted in the release of significant amounts of MV from all three cells and were in line with those previous reported by us [22]. In addition, pre-incubation of the cells with either cycloheximide or actinomycin D, did not significantly alter the release of MV from either of the cell lines tested (Figure 3). However, pre-incubation of cells with cycloheximide significantly reduced the release of TF antigen within the released MV (Figure 4) compared to that from the PAR2-activated cells pre-incubated with the vehicle. The reduction in the incorporation of TF antigen into the released MV was reflected in thrombin generation potential which was comparable to the non-activated cells (Figure 5). In contrast, pre-treatment of cells with actinomycin D prior to activation was ineffective in altering either the level of MV-associated TF antigen, or the associated thrombin generation activity of the released MV (Figure 4 & 5). Moreover, since the amounts of released MV from activated cells (Figure 3) did not vary considerably between the three treatments, the calculated values for TF antigen or TF activity, per density of MV was proportionally lower in the sample treated with cycloheximide. Detectable amounts of mRNA were not present in any of the isolated MV samples. Finally, comparison of the amounts of TF mRNA, in non-activated and PAR2-activated cells using RT-qPCR, did not indicate further mRNA transcription during the experiment (Table 1).
4. Discussion
The elevated risk of cancer-associated thrombosis is due to multiple risk factors (patient-related, cancer type-related and treatment-related) [3, 29, 30] and may contribute to the ambiguity of findings of existing studies. Even the definition of ‘active cancer’ in major CAT studies is inconsistent and can include patients with fully resected malignancy, or only with a history of cancer [31]. In this study, an effort was made to reduce all recognisable non-cancer related risk factors for VTE prior to examining any association between the expression of TF mRNA levels in tumours and occurrence of IPE. The study was built on our previous in vitro observations [22] and hypothesised that following cellular triggers including PAR2 activation, tumour cells are capable of bursts of expressing and releasing substantial amounts of TF protein (Figure 6). We propose that the presence of such latent reservoirs of TF mRNA may be a precursor for rapid protein production, and therefore a predictor for PE. By quantifying the amount of TF mRNA rather than relying on relative amounts, we detected values in the PE group that were up to five orders of magnitude higher than the no-PE group, without any apparent overlap between the two groups.
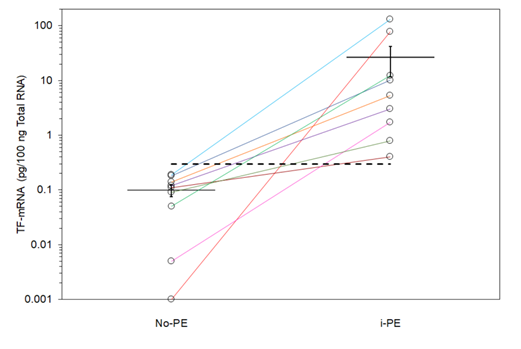
Figure 2: Analysis of the absolute amounts of TF mRNA in the samples with IPE and no PE. Total mRNA was extracted from FFPE tissue samples and the absolute amount of TF mRNA determined using a standard curve prepared using in vitro-transcribed TF mRNA. Matched samples were plotted on a logarithmic scale are shown with separate coloured lines. Dotted line represents the threshold for positive incidence, calculated using ROC analysis.
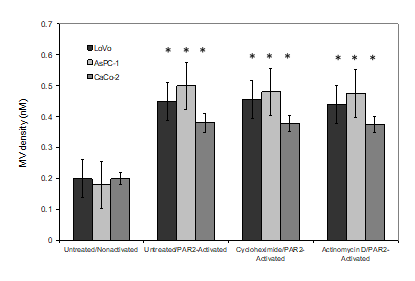
Figure 3: Examination of the effects of cycloheximide and actinomycin D on the release of MV from cells. LoVo, CaCo-2 and ASPC-1 cell lines incubated with cycloheximide (10 nM), excess actinomycin D (1 µM) or the vehicle DMSO (0.02% w/v) before activation with SLIGKV; (20 μM). Conditioned media was collected after 30 min and MV densities were quantified using the Zymuphen MP assay kit. (n=3; *=p<0.05 vs. the respective non-activated sample).
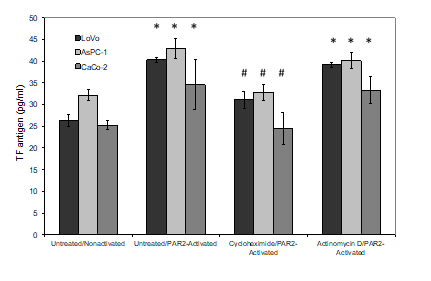
Figure 4: Examination of the effects of cycloheximide and actinomycin D on the incorporation of TF antigen into MV. LoVo, CaCo-2 and ASPC-1 cell lines were treated and activated as described in figure 3. Conditioned media was collected after 30 min and TF antigen levels were measured in the released samples using a TF-ELISA kit. (n=3; *=p<0.05 vs. the respective non-activated sample, #=p <0.05 vs. the respective untreated/PAR2-activated sample).
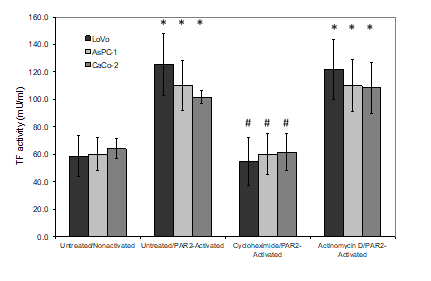
Figure 5: Examination of the effects of cycloheximide and actinomycin D on TF activity associated with released MV. LoVo, CaCo-2 and ASPC-1 cell lines were treated and activated as described in figure 3. Conditioned media was collected after 30 min and and MV were then adsorbed onto the annexin v-coated plates (Zymuphen) and the TF activity was measured in the released samples using thrombin-generation assay. (n=3; *=p<0.05 vs. the respective non-activated sample, #=p <0.05 vs. the respective untreated/PAR2-activated sample).
|
Cell line |
Non-activated |
PAR2-activated |
|
LoVo |
1±0.1 |
1.04±0.35 |
|
AsPC-1 |
1±0.1 |
1.03±0.31 |
|
CaCo-2 |
1±0.2 |
0.94±0.38 |
Table 1: Ratio of TF mRNA in cell lines before and after PAR2 activation. Cell lines were activated using PAR2-agonist peptide (20 µM). The cells were then lysed, total RNA isolated and the relative amounts of TF mRNA determined. (n = 3).
|
TF mRNA |
||
|
(pg/100 ng total RNA) |
Sensitivity |
1-Specificity |
|
-1 |
1 |
1 |
|
0.0025 |
0.889 |
1 |
|
0.0275 |
0.778 |
1 |
|
0.07 |
0.667 |
1 |
|
0.1 |
0.556 |
1 |
|
0.115 |
0.444 |
1 |
|
0.13 |
0.333 |
1 |
|
0.16 |
0.222 |
1 |
|
0.185 |
0.111 |
1 |
|
0.295 |
0 |
1 |
|
0.5925 |
0 |
0.889 |
|
1.2575 |
0 |
0.778 |
|
2.39 |
0 |
0.667 |
|
4.195 |
0 |
0.556 |
|
7.73 |
0 |
0.444 |
|
1.18 |
0 |
0.333 |
|
44.76 |
0 |
0.222 |
|
104.355 |
0 |
0.111 |
Table 2: The sensitivity and specificity results as determined using the ROC analysis. ROC analysis of TF mRNA showed an area under the curve of 1.00; significance <0.001 and CI of 95% (1.00). A potential predictive cut-off concentration for the risk of IPE was selected at 0.295 pg/100ng-total RNA (highlighted in grey) which produced a sensitivity value of 100% towards the incidence of IPE, with a specificity value of 100%.
A potential predictive cut-off concentration for the risk of IPE was selected at 0.295 pg/100ng-total RNA (shown as the dashed line in Figure 2) which produced a sensitivity value of 100% towards the incidence of IPE, with a specificity value of 100% (highlighted in Table 2). Therefore, our data is the first to demonstrate a significant association between the risk of incidentally diagnosed PE in GI cancer and the potential of cancer tissues to express TF mRNA. Circulating cell-derived microvesicles carry TF protein embedded within negatively-charged phospholipids. However, the difficulties in quantifying the release of tumour-derived TF microvesicles at the ideal time of incidence are self-evident and variations may also arise from the clearance of TF-microvesicles by the physiological process [32-34]. The correlation between mRNA and protein expression depends on various biological factors and is not always straightforward [35]. Therefore, it is possible that high levels of mRNA act as a reservoir permitting rapid translation of the protein [36]. This was evident by the reliance of the TF-MV release on protein translation but not mRNA transcription in cell lines examined
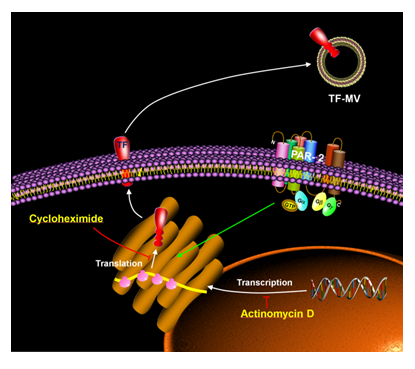
Figure 6: Schematic representation of the mechanism of rapid TF synthesis and release and the inhibition by cycloheximide. Activation of PAR2 on cells leads to rapid translation of TF mRNA into protein and release into TF-MV. This is shown using cycloheximide which inhibits translation and prevents TF-MV release but not by actinomycin D which inhibits mRNA transcription and does not inhibit TF-MV release.
since the blocking of the either protein translation or mRNA transcription did not alter the release of MV per se (Figure 6). This further supports rapid protein synthesis as a source of TF protein following stimulation. In fact previous indications suggest that the percentage increases in the TF release following activation appear to inversely correlate with the ability of cells to release TF under resting condition [22]. Therefore, the level of cellular TF protein stored within the cells may be a function of the turnover of TF, determined by both the expression of TF mRNA and TF protein release from the cells. In contrast, the presence of a reservoir of TF mRNA within the cell indicates the potential for amplified translation and release of the TF protein at an accelerated rate. Few studies have explored the significance of TF mRNA expression, in relation to clinico-pathologic characteristics. However, studies have demonstrated a correlation between TF mRNA expression and the stage of cancer progression [6]. In our proposed model, the amplified incorporation of TF within MV and the rapid release from the cells is dependent on the translation of the protein from accumulated quantities of TF mRNA. Under these circumstances a suitable trigger may promote the rapid, short-term burst in the release of procoagulant microvesicles which can precipitate in thrombus formation, but are then also rapidly cleared from the circulation.
In our study, no difference in PAR2 expression between the two groups of tumour samples was observed. The average normalised Ct value of PAR2 mRNA was 36.85±1.88 for tissue samples from patients with IPE, compared to 36.25±2.87 for those without. However, PAR2 acts as the trigger for TF expression and release in the proposed model [22] and not the cargo that is loaded into the released microvesicles. Consequently, while a limited amount of cell-surface PAR2 may be capable of activating this mechanism, only cells containing large amounts of TF mRNA are capable of producing large procoagulant potentials. Therefore, while tissue biopsies represent the state of the tumour at a single time-point, time-dependant laboratory experimentation permits the effective exploration of the triggering influences and allow the quantification of the fluctuating indicators such as the activation of PAR2, which act as transitory biological processes.
4.1. Limitation of the study
Limitations of this pilot study include, the cohort being generated from IPE patients and perhaps not reflecting the pathophysiological processes of ‘suspected PE’ cases or patients with DVT. We did however exclude all distal (segmental/subsegemental) cases where most of the controversy relating to spurious or false-positive cases arise. It has been suggested that spurious results may arise due to the efficient detection procedures [37] and that some of the distal thrombi may in fact be as a result of various physiopathological factors. Therefore, in order to prevent misrepresentations from the spuriously identified clots, we also excluded these subjects. Once the exclusion criteria were applied a database of 234 cases was not adequate to generate the 50 study subjects hence the study was smaller than originally planned. However, the ‘purist’ approach of the study has generated some insight into the inconsistent literature surrounding TF and VTE prediction. In addition, we cannot extend this observation beyond GI malignancies and finally we cannot confirm that the metastases present at the time of IPE still retained the pathological characteristics of the primary cancer that generated them and which was the subject of the analyses.
5. Conclusions
In conclusion, the release of TF protein within MV, from activated cancer cells appears to be regulated at the protein translation levels rather than de novo mRNA expression. The higher levels of TF mRNA stored within the tumours may act as latent sources for TF expression which may be retained within tumour cells with substantially increased potential for rapid expression and release of TF into the blood. Consequently, the quantity of TF mRNA expressed by the tumour may be a determinant risk of IPE in patients with GI malignancies.
Authors' contributions
The study was designed by AM and CE, and the experimental work was carried out by NEB, SF, JC and CE. The data were evaluated by CE, NEB, FH and AM and the manuscript was prepared by CE, NEB, FH, MJJ and AM. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Ethical review and approval were waived for this study, since ethical approval was obtained from IRAS (project ID: 185389).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111 (2008): 4902-4907.
- Lyman GH, Eckert L, Wang Y, et al. Venous thromboembolism risk in patients with cancer receiving chemotherapy: a real-world analysis. Oncologist 18 (2013): 1321-1329.
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol 27 (2009): 4839-4847.
- Kakkar AK, Lemoine NR, Scully MF, et al. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg 82 (1995): 1101-1104
- Ueno T, Toi M, Koike M, et al. Tissue factor expression in breast cancer tissues: its correlation with prognosis and plasma concentration. Br J Cancer 83 (2000): 164-170
- van den Berg YW, Osanto S, Reitsma PH, et al. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood 119 (2012): 924-932
- Callander NS, Varki N, Rao LV. Immunohistochemical identification of tissue factor in solid tumors. Cancer 70 (1992): 1194-1201
- Nakasaki T, Wada H, Shigemori C, et al. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol 69 (2002): 247-254
- Poon RT, Lau CP, Ho JW, et al. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin Cancer Res 9 (2003): 5339-5345.
- Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA 97 (2000): 5255-5260.
- Collier ME, Ettelaie C. Regulation of the incorporation of tissue factor into microparticles by serine phosphorylation of the cytoplasmic domain of tissue factor. J Biol Chem 286 (2011):11977-11984.
- Wojtukiewicz MZ, Hempel D, Sierko E, et al. Protease-activated receptors (PARs)–biology and role in cancer invasion and metastasis. Cancer Metastasis Rev 34 (2015): 775-796.
- Ünlü B, Versteeg HH. Effects of tumor-expressed coagulation factors on cancer progression and venous thrombosis: is there a key factor? Thromb Res 133 (2014): S76-84.
- Darmoul D, Gratio V, Devaud H, et al. Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol 162 (2003): 1503-1513.
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 78 (1999): 285-291.
- Thaler J, Preusser M, Ay C, et al. Intratumoral tissue factor expression and risk of venous thromboembolism in brain tumor patients. Thromb Res 131 (2013): 162-165.
- Hernández C, Orbe J, Roncal C, et al. Tissue factor expressed by microparticles is associated with mortality but not with thrombosis in cancer patients. Thromb Haemost 110 (2013): 598-608.
- Thaler J, Koder S, Kornek G, et al. Microparticle-associated tissue factor activity in patients with metastatic pancreatic cancer and its effect on fibrin clot formation. Transl Res 163 (2014): 145-150.
- Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. British Journal of Cancer 102 (2010): S2-9.
- Khorana AA, Kamphuisen PW, Meyer G, et al. Tissue Factor As a Predictor of Recurrent Venous Thromboembolism in Malignancy: Biomarker Analyses of the CATCH Trial. J Clin Oncol 35 (2017): 1078-1085.
- Zwicker JI, Liebman HA, Bauer KA, et al. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study). Br J Haematol 160 (2013): 530-537.
- Ettelaie C, Collier M, Featherby S, et al. Analysis of the potential of cancer cell lines to release tissue factor-containing microvesicles: correlation with tissue factor and PAR2 expression. Thrombosis Journal 14 (2016): 2.
- Iablokov V, Hirota CL, Peplowski MA, et al. Proteinase-activated receptor 2 (PAR2) decreases apoptosis in colonic epithelial cells. J Biol Chem 289 (2014): 34366-34377.
- Bozas G, Jeffery N, Ramanujam-Venkatachala D, et al. Prognostic assessment for patients with cancer and incidental pulmonary embolism. Thrombosis Journal 16 (2018): 8.
- Ettelaie C, Fountain D, Collier ME, et al. Low molecular weight heparin downregulates tissue factor expression and activity by modulating growth factor receptor-mediated induction of nuclear factor-κB. Biochim Biophys Acta 1812 (2011): 1591-1600.
- Ettelaie C, Collier ME, Maraveyas A, et al. Characterization of physical properties of tissue factor-containing microvesicles and a comparison of ultracentrifuge-based recovery procedures. J Extracell Vesicles 3 (2014).
- Madkhali Y, Featherby S, Collier ME, et al. The Ratio of Factor VIIa:Tissue Factor Content within Microvesicles Determines the Differential Influence on Endothelial Cells. TH Open 3 (2019): e132-e145.
- Ettelaie C, Su S, Li C, et al. Tissue factor-containing microparticles released from mesangial cells in response to high glucose and AGE induce tube formation in microvascular cells. Microvasc Res 76 (2008): 152-160.
- Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost 117 (2017): 219-230.
- Moik F, Ay C, Pabinger I. Risk prediction for cancer-associated thrombosis in ambulatory patients with cancer: past, present and future. Thromb Res 191 (2020): S3-S11.
- Frere C, Crichi B, Lejeune M, et al. Are patients with active cancer and those with history of cancer carrying the same risks of recurrent VTE and bleeding while on anticoagulants? Cancers (Basel) 12 (2020): 917.
- Collier ME, Mah PM, Xiao Y, et al. Microparticle-associated tissue factor is recycled by endothelial cells resulting in enhanced surface tissue factor activity. Thromb Haemost 110 (2013): 966-976.
- Dasgupta SK, Le A, Chavakis T, et al. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation 125 (2012): 1664-1672.
- Kawamoto T, Ohga N, Akiyama K, et al. Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS One 7 (2012): e34045.
- Maier T, Güell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett 583 (2009): 3966-3973.
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13 (2012): 227-232.
- Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. Brit Med J 347 (2013): f3368.
