Tolbutamide Eye Drops Increase Aqueous Humor Outflow and Lower Intraocular Pressure: A Proof of Concept for Glaucoma Treatment
Article Information
Gabriele Thumann1,2*, Nino Sorgente3, Martina Kropp1,2 and Christian Paul Jonescu-Cuypers1,2
1Experimental Ophthalmology, University of Geneva, 1205 Geneva, Geneva, Switzerland.
2Department of Ophthalmology, University Hospitals of Geneva, 1205 Geneva, Geneva, Switzerland.
2Elements Pharmaceuticals, Inc., Washington DC, USA.
*Corresponding Author: Gabriele Thumann. Department of Ophthalmology, University Hospitals of Geneva, 1205 Geneva, Geneva, Switzerland
Received: 06 April 2021; Accepted: 15 April 2021; Published: 25 April 2021
Citation: Gabriele Thumann, Nino Sorgente, Martina Kropp and Christian Paul Jonescu-Cuypers. Tolbutamide Eye Drops Increase Aqueous Humor Outflow and Lower Intraocular Pressure: A Proof of Concept for Glaucoma Treatment. Journal of Ophthalmology and Research 4 (2021): 114-127.
View / Download Pdf Share at FacebookAbstract
Background: Glaucoma refers to a heterogeneous group of diseases characterized by retinal cell degeneration and optic nerve atrophy leading to blindness. Even though about 40% of patients have normal intraocular pressure (IOP), current treatment. focuses on lowering IOP. With time, current drugs become less effective, which has motivated the search for novel drugs.The objective was to establish whether modulators of ATP-sensitive potassium channels influence IOP.
Methods: The double-blind, 5-day short duration Proof-Of-Concept study was carried out at the Ophthalmology Clinic, University of Cologne, Germany. The only inclusion criteria were a diagnosis of glaucoma, ability to understand that they would be treated with an experimental drug, and readiness to sign a consent form.
Results: In rabbits, 1 h after topical application of 80 µL of 0.5% tolazamide, tolbutamide, glibenclamide, and chlorpropamide suspended in phosphate buffered saline IOP de-creased, whereas 0.5% diazoxide increased IOP. In Cynomolgus monkeys tolbutamide decreased IOP. In 9 glaucoma patients treated for 5 days with one drop of a 0.5% tolbutamide solution twice daily, IOP was an average of 17% lowered. In one patient with ocular hypertension, tolbutamide lowered IOP by a 5-day average of 29% and increased aqueous humor outflow by 185%. No local adverse effects were observed.
Conclusions: The data presented show that blockers of the ATP-sensitive potassium channels lower IOP whereas diazoxide, an ATP-sensitive potassium channel opener, increases IOP suggesting that elevated IOP results from an ionic imbalance. The data suggest that sulfonylurea drugs are useful for the treatment of glaucoma.
Keywords
Glaucoma, open-angle glaucoma, tolbutamide, ATP-sensitive potassium channels, ATP-sensitive potassium channel opener, ATP-sensitive potassium channel blocker, intraocular pressure, aqueous humor outflow
Glaucoma articles, open-angle glaucoma articles, tolbutamide articles, ATP-sensitive potassium channels articles, ATP-sensitive potassium channel opener articles, ATP-sensitive potassium channel blocker articles, intraocular pressure articles, aqueous humor outflow articles
Article Details
1. Introduction
Glaucoma refers to a heterogeneous group of ocular diseases that are characterized by retinal ganglion cell (RGC) degeneration [1] and progressive optic nerve atrophy; left untreated glaucoma gradually leads to visual field loss and blindness. Although research is considerable, the pathological mechanisms involved in the onset and development of glaucoma are not understood. Primary open angle glaucoma (POAG), the most common type, is associated with progressive loss of RGC axons, along with supporting glia and vasculature, resulting in degeneration of the optic nerve head and loss of peripheral vision [2].
Elevated intraocular pressure (IOP) is considered the main risk for the onset and progression of POAG, even though about 40% of patients present IOP values within the normal range [3, 4] suggesting that elevated IOP is neither essential nor sufficient [5, 6] to cause glaucoma; in fact, the risk of unilateral blindness in POAG patients treated to lower IOP is estimated to be about 27% [7] indicating that lowering IOP retards but does not prevents RGC degeneration and blindness.
Since elevated IOP, which results from impaired drainage of aqueous humor through the trabecular meshwork-Schlemm’s canal complex [8], is the only modifiable risk factor, current therapies seek to lower IOP even in patients with normal IOP. Even though effective surgical procedures have been developed to lower IOP, pharmaceutical therapy with drugs administered topically is the accepted management of glaucoma.
Topical prostaglandin analogs are most frequently used to treat glaucoma. Used once daily, prostaglandin analogs lower IOP by 25-30% [9] and stabilize it at a lower level by increasing uveoscleral outflow [10] without significant systemic side effects; however, they have considerable local adverse reactions [11-15] and in rare cases cause cystoid macular edema [16]. Several drugs belonging to various chemical classes is also used to treat glaucoma; however, side effects have limited their use as first line treatment [17-21]. Current pharmacological agents are generally effective at lowering IOP but in up to 40% of patients, monotherapy does not provide sufficient control of IOP and a combination of drugs is required, reducing compliance, hand increasing side effects [22-24].
Side effects and patients becoming unresponsive to current drugs while neurodegeneration continues, albeit at a lower rate [25], has instigated the search for pharmacological agents with novel mode of action that improve aqueous humor outflow via the conventional trabecular meshwork-Schlemm’s canal pathway. Netarsudil dimesylate, which lowers IOP by increasing outflow facility and lowering episcleral venous pressure [26], was approved in 2017.
The precise cellular defect responsible for glaucoma is unknown; however, since water dynamics are linked to ion transport, it can be assumed that ion transport in the trabecular meshwork, ciliary body, and retina are involved in water dynamics and glaucoma. Chiang and Lin reported that cromakalim, an ATP-sensitive potassium channel (KATP) opener, increased IOP in rabbits [27]; however, Chowdhury et al. have reported that in mice, rats, and isolated human anterior segments, cromakalim lowers IOP [28, 29]. Panchal and colleagues have reported that nicorandil and pinacidil, ATP-sensitive channel openers, counteracted the IOP rise in an acute model of glaucoma [30].
KATP channels are involved in numerous cellular functions from insulin secretion to natriuresis [31-35]. It is generally accepted that activation of KATP channels leads to K+ efflux, vasodilation, membrane hyperpolarization, and decreases Ca2+ entry into cells whereas inhibition of KATP channels causes membrane depolarization, vasoconstriction, and increases Ca2+ entry into cells [36]. In fact, KATP channel inhibitors, such as glibenclamide, reduce vasogenic and other types of edema [37, 38]. In view of the contradictory results of Chang and Chowdhury and the role of KATP inhibitors in reducing edema we revisited the effect of KATP channel modulators on IOP and aqueous humor dynamics in the eye. Here, we report that KATP channel inhibitors lower IOP in rabbits and that tolbutamide lowers IOP in Cynomolgus monkeys, in normal and glaucoma subjects, and increases aqueous humor flow and outflow with a significant net increase in outflow.
2. Results
2.1 Effect of KATP Modulators and Timolol on IOP of Rabbits
At 1-hour post drug administration timolol decreased IOP by 15% (n=25), tolazamide decreased IOP by 16% (n=10), tolbutamide decreased IOP by 14% (n=5), glybenclamide decreased IOP by 8% (n=20), and diazoxide increased IOP by 25% (n=5) compared to control rabbits instilled with PBS (n=25). At 2 hours IOP was only 3% lower than before administration of the drug for timolol and for glibenclamide, 6% lower for tolbutamide and tolazamide, and 22% higher for diazoxide. No redness, irritation, or behavioral changes were noted in the animals instilled with PBS or any of the drugs.
2.2 Effect of Tolbutamide On IOP Of Cynomolgus Monkeys
The IOP in the two monkeys treated with tolbutamide in suspension decreased by an average of 7.5 mm Hg or 31% and in the two monkeys treated with 0.5% solution the IOP decreased by an average of 6 mm Hg or 26% from the pre-treatment values. In the monkeys treated with vehicle the IOP decreased by 4.5 mm Hg or 19%, which was likely the result of the anesthesia [39]. No evidence of irritation, or redness were noticed in the 4 monkeys.
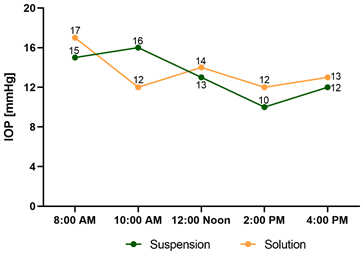
2.3 Effect of Sodium Tolbutamide on the IOP of a Normal Subject
Figure 1 shows that tolbutamide in suspension and solution, decreased IOP in a normal subject; considering that in humans the highest IOP is at 12:00 Noon 40 the IOP de-crease observed is an effect of tolbutamide and not due to diurnal variations. Sodium tolbutamide solution was chosen for additional studies to avoid the possible dose un-certainty when administering a suspension. No burning, irritation, or redness were observed.
2.4 Effect of 0.5%Tolbutamide in Human Subjects with Elevated IOP
Both eyes of a patient with pseudoexfoliative glaucoma and of a patient with POAG were treated with one drop of 0.5% tolbutamide solution. IOP was measured at the times indicated in Table 1 by a nurse while the patients were hospitalized. Table 1 shows that 5 hours after administration of tolbutamide, IOP was substantially lower in both eyes of the patient with pseudoexfoliation glaucoma. In the POAG patient tolbutamide decreased IOP in both eyes as early as 1 hour and was meaningfully lower at 3- and 16-hours post-administration.
2.5 Effect of 0.5% Tolbutamide on Human Subjects During a 5-Day Treatment
To deter-mine the longer-term effect of tolbutamide on IOP and possible side effects, 9 patients with diagnosed POAG were treated with one drop of 0.5% tolbutamide twice daily for 5 days. Table 2 shows that in seven of the nine POAG patients, treatment with one drop of 0.5% tolbutamide decreased IOP in the treated eye by a 5-day average of 4.91 mm Hg (range 9.5 to 2.3 mm Hg) compared to an average of 0.7 mm Hg (range 0.1 to 5.4 mm Hg) for the untreated eye. In patients 3 and 7, IOP decreased in both eyes suggesting that the patients may have used the drug in both eyes. Patient 3 showed an average 5-day decrease of 7.1 mm Hg in the treated eye; however, the treated eye showed no or a small decrease on days 1, 2 and 3 and a large decrease on days 4 and 5 (10-12 mm Hg); the control eye showed a significant decrease on days 2, 4 and 5 suggesting that the patient may have instilled the drug to both eyes on some days.
2.6 Effect of Tolbutamide on the IOP of a 42-Year-Old Female Ocular Hypertensive Tension (OHT) Subject without Visual Field Loss
Table 3 shows that during the 5-day treatment, the IOP in the tolbutamide-treated eye ranged from a high of 22 mm Hg at 9:00 AM on day 2 to a low of 10 mm Hg on day 9. The average 5-day IOP in the tolbutamide- treated eye was -6.1 ± 3.8 mm Hg (33%) lower than the pre-treatment IOP and significantly different (p=0.0001) from the 5-day mean (+0.5 ± 2.1) of the vehicle-treated eye.
2.7 Effect Of 0.5% Tolbutamide On Aqueous Humor Outflow Facility
Table 4 shows that tolbutamide increases both aqueous humor production and outflow. Production in-creased by 158% whereas outflow increased by 342%, a net 185% increase in outflow. In the untreated eye both flow and outflow decreased from the pre-treatment values, which may be the result of diurnal fluctuations [42, 43].
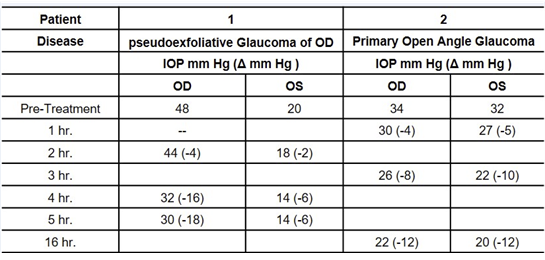
Table 1: Effect of one drop of 0.5% sodium tolbutamide on the IOP of human subjects.
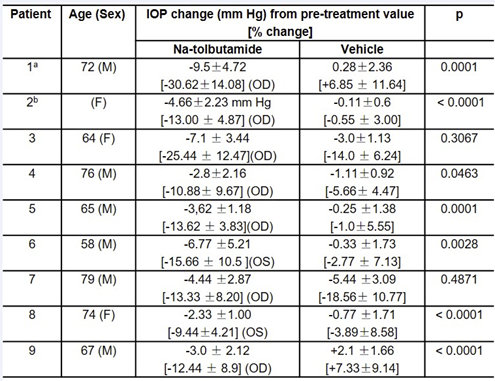
Table 2: Mean 5-Day effect of 0.5% sodium tolbutamide on IOP of 9 glaucoma patients.
(a) Patient 1 was hospitalized for conditions unrelated to glaucoma; drops were administered by a nurse. The patient, the nurse, and the doctor measuring IOP were not aware of which eye received the drug and which the vehicle.
(b) Age not recorded.
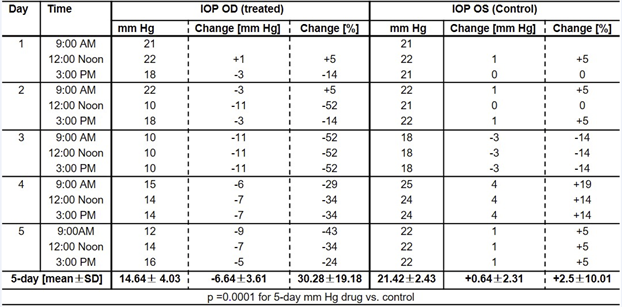
Table 3: Effect of 0.5% sodium tolbutamide on IOP of an OHT patient.
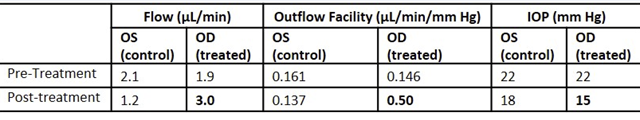
Table 4: Effect of tolbutamide on inflow and outflow of aqueous humor in an OHT patient.
3.Discussion
Glaucoma is a complex disease that presents significant obstacles to treatment because over time many patients require a combination of more than one drug to control IOP and even when IOP is controlled, optic nerve degeneration continues at a slower rate [41, 42].
When IOP can no longer be controlled with medications surgical intervention is required, which often is not a permanent solution [43]. It is, therefore, essential to develop novel drugs that modulate the metabolic processes of trabecular meshwork, ciliary body, and retinal cells.
Here, we have shown that in rabbits blockers of KATP channels, decrease IOP whereas the KATP channel opener diazoxide elevates IOP. In monkeys, tolbutamide in both in solution and suspension also lowered IOP.
Since tolbutamide has been used as a systemic drug to treat diabetes for over 50 years without severe side effects at doses ranging from 250 mg to 3 g/day and since we did not observe any discomfort in animals treated with tolbutamide, we examined the effect of 0.5% tolbutamide on the IOP of a normal subject and of glaucoma patients. One drop of tolbutamide administered topically to a normal subject, to a pseudoexfoliative glaucoma, and to a POAG patient lowered IOP by over 20%. In seven of nine POAG patients 0.5% tolbutamide applied twice daily for 5 days there was a statistically significant drop in the 5-day average IOP (p ≥ 0.05). In two patients, IOP decreased significantly in both eyes, suggesting that tolbutamide was instilled in both eyes. Analysis of aqueous humor dynamics showed that tolbutamide increased both formation and outflow; however, the outflow increased considerably more such that there was a net outflow increase of 200%. It is noteworthy that no burning, irritation, or redness were observed or reported by any patient. Since sulfonylureas bind tightly to plasma proteins when administered systemically [44, 45] and may not pass the brain-blood barrier [46], this indicates that systemic administration would not be useful for the treatment of glaucoma. In the pancreas, tolbutamide inhibits the opening of KATP channels resulting in membrane depolarization, voltage activated calcium channels opening, Ca+2 influx into beta cells and insulin secretion [31]. KATP channels in their various isoforms are found in most cells and tissues, where the various isoforms confer upon the channels specific properties that regulate the tissue metabolic activities [47-55].
Our results showing that KATP channel inhibitors lower IOP, increase aqueous formation and outflow are contrary to those of Chowdhury et al. who have reported that KATP channel openers lower IOP and increase aqueous outflow in mice and rats [28, 29]. There are differences between our studies and those of Chowdhury et al. specifically, we used rabbits, Cynomolgus monkeys and human subjects, Chowdhury et al. used mice, rats and isolated human anterior segments. We formulated the drugs in PBS, pH 7.4, or an acceptable vehicle for ophthalmic human use; Chowdhury et al. used DMSO and polyethoxylated castor oil. Whether formulation differences or species differences account for the contradictory results will require additional studies.
4. Materials and Methods
The use of animals and all treatment protocols were approved by the responsible office for animal protection and adhered to the ARVO statement for the use of Animals in ophthalmic and vision research.
4.1. Effect of KATP Modulators on IOP of Rabbits New Zealand White rabbits (2-3 kg), were instilled in the right eye with sterile PBS (n=25), 0.5% timolol (n=25), 0.5% glibenclamide (n=20), 0.5% tolazamide (n=10), 0.5% tolbutamide (n=5), 0.5% chlorpromazine (n=2), and 0.5% diazoxide (n=5). All drugs were suspended in sterile PBS, pH 7.4; 80 µl of each drug were instilled in two 40 µL doses at an interval of 2 minutes. IOP was measured using a Tonopen at 1 and 2 hours after the administration of the drug. The rabbit studies were done by Dr. Weiping Yang on awake rabbits (Aviva Research Inc., San Diego, CA, USA).
4.2. Preparation of Tolbutamide Solution for use in Cynomolgus Monkeys
Tolbutamide, was converted into the water-soluble sodium salt by dissolving it in 0.25 M NaOH; the dissolved drug was added to 0.4% HPMC, the pH was adjusted to 6.7, tonicity to 300 mOsm with NaCl, and preserved with 0.001% thimerosal. The effect of 0.5% solubilized tolbutamide on IOP was compared to 0.5% tolbutamide suspended in PBS in Cynomolgus monkeys.
4.3. Preparation of Tolbutamide Solution for use in Human Subjects
Sodium tolbutamide was formulated as a 0.5% solution in 1.5% boric acid, 3% povidone, 0.002% thimerosal, pH 6.5, 281 mOsm. The drug was prepared under GMP guidelines by SK Pharmaceuticals (San Diego, CA, USA). Drug and vehicle control vials were color coded. Control vials were prepared in like manner without tolbutamide.
4.4 Effect of Tolbutamide, a KATP Inhibitor on IOP of Cynomolgus Monkeys
Monkeys were anesthetized with ketamine hydrochloride (25 mg/kg) and baseline IOP determined. One drop of 0.5% tolbutamide suspension and one drop of 0.5% tolbutamide solution were each administered to the right eye of two monkeys; vehicle was administered to the left eyes. IOP was determined one hour after drug or vehicle administration. We are grateful to Dr. Craig Crosson for performing the study at Texas Tech University Health Sciences Center (Lubbock, Texas, USA).
4.5 Effect of Tolbutamide on IOP in Human Subjects
The human study was done with the approval of the Ethics Committee of the University of Cologne (Germany) and with the informed consent of each patient.
4.6 Effect of Tolbutamide on IOP of a Normal Subject
One drop of 0.5% tolbutamide suspended in PBS was instilled into the right eye and one drop of 0.5% tolbutamide solution was instilled into the left eye of a male, 62-year-old subject at 7:30 AM; IOP was measured by applanation tonometry at 8:00 AM and at 2-hour intervals until 4:00 PM.
4.7 Effect of Tolbutamide on IOP of Glaucoma Patients
Both eyes of a pseudoexfoliative glaucoma patient and of a POAG patient, hospitalized for conditions unrelated to glaucoma, were treated with one drop of 0.5% tolbutamide and IOP measured at “0” time and at the indicated times (Fig. 1 and Table 1) by applanation tonometry.
4.8 Effect of 5-Day Treatment with 0.5% Tolbutamide on the IOP of Glaucoma Patients
To determine whether longer-term treatment with tolbutamide lowered IOP, nine POAG patients (58-79 years of age; 3 females and 6 males) were treated with one drop of 0.5% tolbutamide twice daily. Patients were given and instructed to instill one drop from the vial with a red label (drug) into a specified eye and one drop with the green label (vehicle) into the contralateral eye at 9:00 AM and 10:00 PM each day; patients were asked to come to the ophthalmology clinic at 8:30 AM and 4:30 PM each day to have the IOP measured at 9:00 AM and at 5:00 PM. Patients and study personnel were not informed which vial contained the drug and which vial contained the vehicle.
4.9 Effect of 5-Day Treatment with 0.5% Tolbutamide on the IOP of an OHT Patient
A 42-year-old female with long-standing OHT without visual field loss, who was hospitalized for a condition unrelated to OHT, was administered 1 drop of 0.5% tolbutamide at 9:00 AM and at 10:00 PM to the right eye and vehicle to the left eye; IOP was measured before administration, at 9:00 AM, 12:00 noon, and 3:00 PM. Medication vials were color-coded, and the patient and study personnel were not informed of which eye received the drug and which eye received the vehicle.
4.10 Effect of 0.5% Tolbutamide on Aqueous Humor Outflow Facility
To define the mechanism of action of tolbutamide, rate of aqueous humor flow and outflow were determined by fluorophotometry in the OHT subject after the patient was discharged from the hospital. The patient was asked to apply one drop of the red-colored vial (tolbutamide) and one drop of the green vial (vehicle) to the left eye and the right eye at 9:15 AM and one at 10:00 PM for 3 days. On the morning of the fourth day the patient was asked to come to the clinic where fluorescein drops (0.25% fluorescein-0.4% benoxinate HCl) were applied to each eye five times during a 30-minute period; five hours after the last drop was applied, fluorescein was measured in the anterior chamber (Fluorotron Master II, Mountain View, CA) every 30 minutes for 2 hours. IOP was measured by applanation tonometry.
4.11 Statistical Analysis
Data was analyzed using the 2-sided paired t-test for 2 dependent means using GraphPad Prism (version 8.0.1, GraphPad software Inc., San Diego, CA, USA). A p-value of ≤ 0.05 was considered significant.
5. Conclusion
We have shown that sulfonylureas administered topically to the eye lower IOP without any observable local side effects at a dose 500-fold lower than the lowest dose results from a greater increase in aqueous humor outflow via the trabecular meshwork than the increased formation; increased formation may represent a reversal of the decreased metabolic activity that occurs with age. The results presented here of the effect of sulfonylureas on IOP and aqueous dynamics are a proof-of-concept and validation on a larger number of glaucoma patients is necessary before conclusions can be reached as to the mechanism of the IOP lowering effect of sulfonylureas.
6. Patents
Patent US No: 10,780,068 “Methods and compositions for improving eye health”, Continuation-in-part application by the same title, application No.:16/938, 628 and PCT/US2018/030988.
Patent No: 5,965,620 and 5,629,345 “Methods and compositions for ATP-sensitive K+ channel inhibition for lowering intraocular pressure”.
Author Contributions
Conceptualization, G.T. and N.S.; Methodology, G.T. and C.P.J.-C.; Formal Analysis, G. T., N.S. and M.K.; Writing – Original Draft Preparation, N.S., M.K. and C.P.J-C.; Writing – Review and Editing, G.T.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are openly available in Yareta at [doi]. 10.26037/ yareta:rfnal 7rkpzamzpcglubitcct6a.
Conflicts of interest
The authors are co-inventors of patent US No: 10,780,068 “Methods and compositions for improving eye health”, and co-inventors of a Continuation-in-part application by the same title, application No.:16/938, 628 and PCT/US2018/030988. Nino Sorgente is the co-inventor on Patent No: 5,965,620 and 5,629,345 “Methods and compositions for ATP-sensitive K+ channel inhibition for lowering intraocular pressure”. Nino Sorgente is President of the newly incorporated Elements Pharmaceuticals.
References
- Vohra R, Tsai JC, Kolko M et al. The role of inflammation in the pathogenesis of glaucoma. Surv Ophthalmol 58 (2013): 311-320.
- Kwon YH, Fingert JH, Kuehn MH, et al. Primary open-angle glaucoma. N Engl J Med 360 (2009): 1113–1124.
- Sommer A, Tielsch JM, Katz J, et al. Relationship Between Intraocular Pressure and Primary Open Angle Glaucoma Among White and Black Americans: The Baltimore Eye Survey Arch Ophthalmol 109(1991): 1090-1095.
- Fan N, Wang P, Tang L, et al. Ocular blood flow and normal tension glaucoma. Biomed Res Int (2015): 308505.
- Thonginnetra O, Greenstein VC, Chu D, et al. Normal versus High Tension Glaucoma: A Comparison of Functional and Structural Defects. J Glaucoma 19 (2010): 151–157.
- Brubaker RF. Delayed functional loss in glaucoma. LII Jackson Memorial Lecture. Am J Ophthalmol 121 (1996): 473?483.
- Hattenhauer MG, Johnson DH, Ing HH, et al. The probability of blindness from open?angle glaucoma. Ophthalmology 105 (1998): 2099?2104.
- Fautsch MP, Johnson DH. Aqueous humor outflow: what do we know? Where will it lead us? Invest Ophthalmol Vis Sci 47 (2006): 4181–4187.
- van der Valk R, Webers CA, Lumley T, et al. Schouten JS. A network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressure. J Clin Epidemiol 62 (2009): 1279-1283.
- Nilsson SF, Drecoll E, Lütjen-Drecoll E, et al. The prostanoid EP2 receptor agonist butaprost increases uveoscleral outflow in the cynomolgus monkey. Invest Ophthalmol Vis Sc.47 (2006): 4042–4049.
- Li F, Huang W, Zhang X. Efficacy and safety of different regimens for primary open-angle glaucoma or ocular hypertension: a systematic review and network meta-analysis. Acta Ophthalmol 96 (2018): e277-e284.
- Holló G. The side effects of the prostaglandin analogues. Expert Opin Drug Saf 6 (2007): 45-52.
- Inoue K, Shiokawa M, Wakakura M, et al. Deepening of the Upper Eyelid Sulcus Caused by 5 Types of Prostaglandin Analogs. J Glaucoma 22 (2013): 626-631.
- Ung T, Currie ZI. Periocular changes following long-term administration of latanoprost 0.005%. Ophthalmol Plast Reconstr Surg 28 (2012): e42-e44.
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg 24 (2008): 302-307.
- Makri OE, Tsapardoni FN, Plotas P, et al. Cystoid macular edema associated with preservative-free latanoprost after uncomplicated cataract surgery: case report and review of the literature. BMC Res Notes 10 (2017): 127.
- Hayreh SS, Podhajsky P, Zimmerman MB. Beta-blocker eyedrops and nocturnal arterial hypotension. Am J Ophthalmol 128 (1999): 301-309.
- Netland PA, Weiss HS, Stewart WC, et al. Cardiovascular effects of topical carteolol hydrochloride and timolol maleate in patients with ocular hypertension and primary open-angle glaucoma. Night Study Group. Am J Ophthalmol 123 (1997): 465-477.
- Schrems WA, Schrems-Hoesl LM, Mardin CY, et al. The Effect of Long-term Antiglaucomatous Drug Administration on Central Corneal Thickness. J Glaucoma 25 (2016): 274-280.
- Wu N, Chen Y, Yu X, et al. Changes in Corneal Biomechanical Properties after Long-Term Topical Prostaglandin. Therapy PLoS One 11 (2016): e0155527.
- Brandt JD, Wittpenn JR, Katz LJ, et al. Conjunctival impression cytology in patients with glaucoma using long-term topical medication. Am J Ophthalm 112 (1991): 297–301.
- Gurwitz JH, Glynn RJ, Monane M, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health 83 (1993): 711-716.
- McKinnon SJ, Goldberg LD, Peeples P, et al. Current management of glaucoma and the need for complete therapy. Am J Manag Care 14(2008): S20-27.
- Hasebe Y, Kashiwagi K, Tsumura T, et al. Changes in adherence and associated factors among patients on newly introduced prostaglandin analog and timolol fixed-combination therapy. Patient Prefer Adherence 12 (2018): 1567-1577.
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 130 (2000): 429-440.
- Kazemi A, McLaren JW, Kopczynski CC, et al. The Effects of Netarsudil Ophthalmic Solution on Aqueous Humor Dynamics in a Randomized Study in Humans. J Ocul Pharmacol Ther 34 (2018): 380-386.
- Chiang C, Lin C. Effects of Cromakalim and Nicorandil on Intraocular Pressure After Topical Administration in Rabbit Eyes. J Ocul Pharmacol Ther 11 (1995): 195–201.
- Chowdhury UR, Bahler CK, Hann CR, et al. ATP-Sensitive Potassium (K ATP) Channel Activation Decreases Intraocular Pressure in the Anterior Chamber of the Eye. Invest Ophthalmol Vis Sci 52 (2011): 6435-6442.
- Chowdhury UR, Holman B, Fautsch MP. ATP-Sensitive Potassium (K(ATP)) Channel Openers Diazoxide and Nicorandil Lower Intraocular Pressure In Vivo. Invest Ophthalmol Vis Sci 54 (2013): 4892-4899.
- Panchal SS, Mehta AA, Santani DD. Effect of potassium channel openers in acute and chronic models of glaucoma. Taiwan J Ophthalmol 6 (2016): 131-135.
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 54 (1989): 87–143.
- Clark MA, Humphrey SJ, Smith MP et al. Unique natriuretic properties of the ATP- sensitive K+-channel blocker glyburide in conscious rats. J Pharmacol Exp Ther 265 (1993): 933–937.
- Ludens JH, Clark M, Lawson A. Effects of a K+ channel blocker on glomerular filtration rate and electrolyte excretion in conscious rats. J Pharmacol Exp Ther 273 (1995): 375–1381.
- Wang T, Wang WH, Klein-Robbenhaar G, et al. Effects of Glyburide on renal tubule transport and potassium-channel activity. Renal Physiol Biochem 18 (1995): 169–182.
- Huang DY, Osswald H, Vallon V. Eukaliuric diuresis and natriuresis in response to the KATP channel blocker U37883A: micropuncture studies on the tubular site of action. Br J Pharmacol 127 (1999): 1811-1818.
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 268 (1995): C799–822.
- Caffes N, Kurland DB, Gerzanich V, et al. Glibenclamide for the treatment of ischemic and hemorrhagic stroke. Int J Mol Sci 16 (2015): 4973-4984.
- Pallan TV, Ahmed I. Glyburide in Treating Malignant Cerebral Edema. Blocking Sulfonyl Urea One (SUR1) Receptors. J Vasc Interv Neurol 7 (2014): 23-25.
- Zuche H, Morinello E, Viestenz A, et al. Reduction of intraocular pressure and ocular pulse amplitude during general anesthesia. Ophthalmologe 112 (2015): 764-769.
- Burfield HJ, Carkeet A, Ostrin LA. Ocular and Systemic Diurnal Rhythms in Emmetropic and Myopic Adults. Invest Ophthalmol Vis Sci 60 (2019): 2237-2247.
- Leske M C, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment. Archives of Ophthalmology 121 (2003): 48–56.
- Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 120 (2002): 1268–1279.
- Liu Q, Saifee M, Yu Y, et al. Evaluation of Long-Term Visual Field Function in Patients Undergoing Glaucoma Drainage Device Implantation. Am J Ophthalmol 216 (2020): 44-54.
- Proks P, Kramer H, Haythorne E, et al. Binding of sulphonylureas to plasma proteins. PLoS One 13 (2018): e019763.
- Szkudlarek A, Pentak D, Ploch A, et al. Effect of Temperature on Tolbutamide Binding to Glycated Serum Albumin. Molecules 22 (2017): pii: E569.
- Lahmann C, Kramer HB, Ashcroft FM. Systemic Administration of Glibenclamide Fails to Achieve Therapeutic Levels in the Brain and Cerebrospinal Fluid of Rodents. PLoS One 10 (2015): e0134476.
- Anagnostis P, Siolos P, Christou K, et al. The effect of antidiabetic medications on the cardiovascular system: a critical appraisal of current data. Hormones (Athens) 17 (2018): 83-95.
- Ashcroft FM, Puljung MC, Vedovato N. Neonatal Diabetes and the KATP Channel: From Mutation to Therapy. Trends Endocrinol Metab 28 (2017): 377-387.
- de Wet H, Proks P. Molecular action of sulphonylureas on KATP channels: a real partnership between drugs and nucleotides. Biochem Soc Trans 43 (2015): 901-907.
- Nichols CG. Adenosine Triphosphate-Sensitive Potassium Currents in Heart Disease and Cardioprotection. Card Electrophysiol Clin 8 (2016): 323-335.
- Tykocki NR, Boerman EM, Jackson WF. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr Physiol 7 (2017): 485-581.
- Aguilar-Bryan L, Clement JP 4th, Gonzalez G, et al. Toward understanding the assembly and structure of KATP channels. Physiol Rev 78 (1998): 227-245.
- Bryan J, Vila-Carriles WH, Zhao G, et al. Toward linking structure with function in ATP-sensitive K+ channels. Diabetes 53 (2004): S104-112.
- Burke MA, Mutharasan RK, Ardehali H. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ Res 102 (2008): 164-176.
- Yang HQ, Subbotina E, Ramasamy R, et al. Cardiovascular KATP channels and advanced aging. Pathobiol Aging Age Relat Dis 6 (2016): 32517.