The Feed of Local Beef Bone Marrow Substitution during Pregnancy affects the Increase in Growth Hormone Offsprings Production of Thirty and Sixty Days Old Mice
Article Information
I Made Tangkas1,4*, Ahmad Sulaeman1, Faisal Anwar1, Agik Suprayogi2, Sri Estuningsih3
1Department of Community Nutrition, Faculty of Human Ecology, Bogor Agriculture University, Bogor, Indonesia
2Department of Anatomy, Physiology and Pharmacology, Faculty of Veterinary Medicine Bogor Agriculture University, Bogor, Indonesia
3Pathology Reproductive Clinic Department, Faculty of Veterinary Medicine Bogor Agriculture University, Bogor, Indonesia
4Department of Chemistry Education, Faculty of Teacher Training and Education Tadulako University of Palu, Palu, Indonesia
*Corresponding Author: I Made Tangkas, Department of Community Nutrition, Faculty of Human Ecology, Bogor Agriculture University, Bogor 16680, Indonesia
Received: 04 October 2019; Accepted: 17 October 2019; Published: 21 October 2019
Citation: I Made Tangkas, Ahmad Sulaeman, Faisal Anwar, Agik Suprayogi, Sri Estuningsih. The Feed of Local Beef Bone Marrow Substitution during Pregnancy affects the Increase in Growth Hormone Offsprings Production of Thirty and Sixty Days Old Mice. Journal of Food Science and Nutrition Research 2 (2019): 316-326.
View / Download Pdf Share at FacebookAbstract
Growth hormone (GH) deficiency during the growth phase will cause the disruption, especially in phases of infant, childhood, and puberty. Local beef bone marrow in Central Sulawesi of Indonesia contains fatty and amino acids, which needed for supporting the proliferation of somatotroph cells of fetal pituitary. This study aims to evaluate the potential of marrow-substituted feed during pregnancy against somatotroph-pituitary cell proliferation seen from the potential of the mice gland that produces the GH. The design of the research uses a complete randomized design (RAL) with a single factor. Fifty female rats and twenty male rats were adapted for two weeks after the estrus was mated in one cage. After the pregnancy, the mother rat was separated into another cage, and then it was intervened with the feed of bone marrow substitution that had been prepared. Then at thirty and sixty days of age, the offsprings were anesthetized with ketamine-xylazine, and the blood was taken from the heart. The GH serum was determined by the ELISA method. The results showed that there were significant differences in the concentration of GH rat-offspring from the mothers fed by the normal feed, IUGR feed, Donggala cattle’s bone marrow-substituted feed, and Bali cattle’s bone marrow-substituted feed, at thirty and sixty days of age (α<0.05).
Conclusion: The local cattle’s marrow from the Donggala or Bali, which is substituted onto the feed then is intervened during the pregnancy can increase the growth hormone production of the offspring born at he age of thirty and sixty days.
Keywords
Bone marrow, Local beef, Growth hormone, Pregnancy intervention
Bone marrow articles, Local beef articles, Growth hormone articles, Pregnancy intervention articles
Bone marrow articles Bone marrow Research articles Bone marrow review articles Bone marrow PubMed articles Bone marrow PubMed Central articles Bone marrow 2023 articles Bone marrow 2024 articles Bone marrow Scopus articles Bone marrow impact factor journals Bone marrow Scopus journals Bone marrow PubMed journals Bone marrow medical journals Bone marrow free journals Bone marrow best journals Bone marrow top journals Bone marrow free medical journals Bone marrow famous journals Bone marrow Google Scholar indexed journals Local beef articles Local beef Research articles Local beef review articles Local beef PubMed articles Local beef PubMed Central articles Local beef 2023 articles Local beef 2024 articles Local beef Scopus articles Local beef impact factor journals Local beef Scopus journals Local beef PubMed journals Local beef medical journals Local beef free journals Local beef best journals Local beef top journals Local beef free medical journals Local beef famous journals Local beef Google Scholar indexed journals Growth hormone articles Growth hormone Research articles Growth hormone review articles Growth hormone PubMed articles Growth hormone PubMed Central articles Growth hormone 2023 articles Growth hormone 2024 articles Growth hormone Scopus articles Growth hormone impact factor journals Growth hormone Scopus journals Growth hormone PubMed journals Growth hormone medical journals Growth hormone free journals Growth hormone best journals Growth hormone top journals Growth hormone free medical journals Growth hormone famous journals Growth hormone Google Scholar indexed journals Pregnancy intervention articles Pregnancy intervention Research articles Pregnancy intervention review articles Pregnancy intervention PubMed articles Pregnancy intervention PubMed Central articles Pregnancy intervention 2023 articles Pregnancy intervention 2024 articles Pregnancy intervention Scopus articles Pregnancy intervention impact factor journals Pregnancy intervention Scopus journals Pregnancy intervention PubMed journals Pregnancy intervention medical journals Pregnancy intervention free journals Pregnancy intervention best journals Pregnancy intervention top journals Pregnancy intervention free medical journals Pregnancy intervention famous journals Pregnancy intervention Google Scholar indexed journals growth hormone articles growth hormone Research articles growth hormone review articles growth hormone PubMed articles growth hormone PubMed Central articles growth hormone 2023 articles growth hormone 2024 articles growth hormone Scopus articles growth hormone impact factor journals growth hormone Scopus journals growth hormone PubMed journals growth hormone medical journals growth hormone free journals growth hormone best journals growth hormone top journals growth hormone free medical journals growth hormone famous journals growth hormone Google Scholar indexed journals puberty articles puberty Research articles puberty review articles puberty PubMed articles puberty PubMed Central articles puberty 2023 articles puberty 2024 articles puberty Scopus articles puberty impact factor journals puberty Scopus journals puberty PubMed journals puberty medical journals puberty free journals puberty best journals puberty top journals puberty free medical journals puberty famous journals puberty Google Scholar indexed journals lactation articles lactation Research articles lactation review articles lactation PubMed articles lactation PubMed Central articles lactation 2023 articles lactation 2024 articles lactation Scopus articles lactation impact factor journals lactation Scopus journals lactation PubMed journals lactation medical journals lactation free journals lactation best journals lactation top journals lactation free medical journals lactation famous journals lactation Google Scholar indexed journals breast milk articles breast milk Research articles breast milk review articles breast milk PubMed articles breast milk PubMed Central articles breast milk 2023 articles breast milk 2024 articles breast milk Scopus articles breast milk impact factor journals breast milk Scopus journals breast milk PubMed journals breast milk medical journals breast milk free journals breast milk best journals breast milk top journals breast milk free medical journals breast milk famous journals breast milk Google Scholar indexed journals
Article Details
1. Introduction
The growth of the fetal organs, the birth weight of the child, and the quality of life of the child in later life determine the success of the pregnancy. A good quality of life can only occur if various factors which involved during the pregnancy, such as maternal nutritional adequacy, maternal nutrition transport to the fetus through the placenta, until the utilization of these nutrients to support fetal growth, are fulfilled. The utilization of nutrition during the pregnancy involves many processes and complex metabolism between the mother, placenta, and fetus. This metabolism is carried out by various enzymes and regulated by many hormones produced by organs or glands.
The mother supplies nutrition and oxygen to the fetus through the placenta, in response to messages sent by the fetus through the placenta. The placenta is a place of exchange (nutrition, oxygen, and metabolic products) between mother and fetus. The adequate trophoblast invasion on the early-pregnancy and the increase of uteroplacental blood flow will ensure the growth of the uterus, placenta, and fetus [1]. The placenta will respond to fetal endocrine signals to improve the transportation of maternal nutrition through placental growth. If the coordination between the mother, placenta, and fetus is disturbed, then as a consequence the growth of the fetal will not be optimum. Next, this growth will affect the short-term and long-term lives of the child born, for example, an increase in mortality and morbidity. Regulation of fetal endocrine growth involves interactions between the mother, the placenta, and the fetus and the hormonal effects caused will determine the fetal long-term physiology program [1]. The results of previous studies have reported that endocrine and cardiovascular system programming occurred during the fetal development, and non-optimum development will happen on intrauterine growth retardation (IUGR) conditions [2].
GH is one of the hormones involved and played a vital role in the phase of marriage preparation and pregnancy. This hormone is composed of one hundred ninety-one amino acids with a molecular weight of 22.124 Dalton, secreted by the somatotropic cells of the pituitary gland [3]. According to Murray et al. [4], human growth starts from the moment of conception and ends in adolescence. During the growth period, it is divided into four phases, namely: fetal growth phase, infant growth phase, childhood growth phase, and puberty growth phase [4]. GH works in stimulating the growth through the growth hormone receptors (GHR) mediator in the cell membrane, and then the interaction of GH and GHR through the endosome pathway causes the translocation of GH and GHR to the nucleus and induces an increase in transcription of a number of genes [5].
In the puberty phase, GH can modulate steroidogenesis, gametogenesis, gonadal differentiation, and increase gonadotropin secretion and response [6]. GH also plays a role in the process that takes place in the mammary and placental glands [7], sexual maturation [8] and ovaries for the occurrence of gametogenesis and steroidogenesis [9]. On animal models research, GH and insulin growth factor-1 (IGF-1), provides stimulant effects on the development of prenatal follicles and proliferation of immature mouse follicle cells [10]. Other research result states that GH interventions in vivo can increase the number of large follicles in pigs [11]. Besides, GH can increase follicular and cell proliferation survival through the increase of the luteinizing hormone activities [12]. GH also has an essential role in oocyte maturation, and this can be seen from the relationship between GH concentrations and the amount of mature oocyte in animals model and humans [13]. Based on the stimulant effects shown by GH on the number and size of follicles, there is an indication that this hormone can increase proliferation cell [14]. Referring to the results of previous studies, GH is a peptide hormone that has a vital role in the preparation and maturation of the reproductive organs.
As a cell that was responsible for the production of GH, which is very needed in the life phase, somatotroph-pituitary should also be able to perform optimal proliferation and maturity, so that the gland can function well after the birth. Under-nutrition experienced by the mother at the time of organogenesis will interfere with cell proliferation and have an impact on the non-optimal maturity of the glands.
The results of nutrition compositions analysis of bone marrow from Donggala and Bali cattle with semi-intensive and traditional livestock practice contain fatty and amino acids that are needed for cell proliferation support. Those fatty acids are omega-3, omega 6, arachidonic acid, DHA, EPA, nervonat, linoleic, α-linolenic acid, oleate, cholesterol. Furthermore, the four types of marrow, which studied in this research, also contain amino acids like isoleucine, threonine, phenylalanine, leucine and lysine, histidine and arginine, tyrosine, serine, valine, protein, aspartate acid, arginine, glycine, and glutamic acid. The purpose of this study is to evaluate the effect of bone marrow-substituted feed from Donggala and Bali cattle with semi-intensive and traditional livestock practice during pregnancy on offspring growth hormone-production.
2. Materials and Methods
The Bone marrow of Donggala and Bali cattle with semi-intensive and traditional livestock practice was obtained from Tavanjuka Palu slaughterhouse, Central Sulawesi Province of Indonesia. This study used fifty female rats and twenty male of two-month old Sprague Dawley strains, obtained from the Center for Tropical Biopharmaca Studies LPPM-IPB. This study was an experimental research with model animals, a single factor, and used a completely randomized design. The feed composition of intervention pregnancy, namely: normal feed (C); intrauterine growth retardation feed (T1); semi-intensive Donggala beef bone marrow-substituted feed (T2); traditional Donggala beef bone marrow-substituted feed (T3); semi-intensive Bali beef bone marrow-substituted feed (T4) and traditional Bali beef bone marrow-substituted feed (T5), referring to the previous study [15]. Interventions and animal research were conducted at the Experimental Animal Laboratory of the Educational Animal Hospital (RSHP) of IPB from December 2017 to April 2018. Stages of research animal trials included the phase of adaptation, intervention, pregnancy testing, pregnancy, birth, and lactation until the offsprings aged thirty and sixty days refer to previous studies [15].
When the rat offsprings aged thirty and sixty days were anesthetized with ketamine-xylazine, the blood was collected from the heart with a 3 ml syringe. Blood serum GH concentration was determined by the ELISA method (Rat GH Elisa Kit 96T Cat. No: E-EL-R0029). The GH concentration data for control and each treatment were analyzed by ANOVA (IBM SPSS Statistics 23). This research has been approved by the Animal Ethics Commission of the Faculty of Veterinary Medicine IPB with SKEH Number: 077/KEH/SKE/XII/
2017.
3. Research Results
Based on the results of serum analysis of thirty and sixty days of age offspring-rat, it is obtained the GH concentrations of rats from the mother given normal feed and intervention feed, as presented in Table 1.
Determination of thirty days of offspring-rat GH was important to do because there was a transition and adaptation process of the offsprings body's physiology, which switched from breast milk to normal feed on these periods. Moreover, in this phase, proliferation cell in various organs or tissues to reach the maturity still happened. The adaptation process can affect somatotroph-pituitary cell proliferation and will affect GH production. The ANOVA analysis of Table 1 showed significant differences in the GH-rat concentrations C, T1, T2, T3, T4, and T5, with F=5.428 and α=0.008 (p<0.05) for the GH concentration of thirty-day-old rats. The result of this analysis indicates that the isocaloric formulation feed during pregnancy affected to the potential of somatotroph-pituitary offspring-rat towards the production of growth hormone.
|
S. No. |
Treatment |
Age (Day) |
|
|
Thirty |
Sixty |
||
|
1 |
C |
22.14 ± 4.860b |
9.61 ± 4.045a |
|
2 |
T1 |
12.21 ± 3.040a |
8.70 ± 0.200a |
|
3 |
T2 |
24.52 ± 6.950c |
9.55 ± 1.410a |
|
4 |
T3 |
20.79 ± 5.515b |
11.61 ± 0.805a |
|
5 |
T4 |
30.38 ± 0.314c |
20.85 ± 5.525b |
|
6 |
T5 |
21.10 ± 2.035b |
16.79 ± 0.385b |
Note: C=offspring from the mother given normal feed (casein 180 g); T1=offspring from the mother which is intervened IUGR-feed (casein 90 g); T2=offspring from the mother who is intervened with the semi-intensive Donggala beef bone marrow substitued formulation feed; T3=offspring from the mother who is intervened with the traditional Donggala beef bone marrow substitued formulation feed; T4=offspring from the mother who is intervened with the semi-intensive Bali beef bone marrow substitued formulation feed; T5=offspring from the mother who is intervened with the traditional Bali beef bone marrow substitued formulation feed; the mean growth hormone concentration with different letters has a significant difference (α<0.05).
Table 1: Average concentration (ng/ml) and deviation standard of growth hormones-rat after thirty and sixty days of age.
Duncan test of GH concentration of thirty day-old offspring-rat shows that there was a significant difference in the concentration of GH rat taken from the mother that were intervened with IUGR feed with those taken from the mother which are given normal feed. This difference was an indication that low protein feed formulation (casein 90 gr) significantly slowed down the somatotroph-pituitary cell proliferation compared to normal feed (casein 180 grams). Then this had an impact on the ability of the gland to produce GH. Furthermore, it was found that there was no significant difference on the concentration of GH between offspring-rats, which were intervened by C feed, and those, which were intervened by T3 and T5. No significant difference was an indication that the bone marrow nutritional component from Donggala or traditional Bali cattle had the same ability to support fetal somatotropic cell proliferation. This effective proliferation on the somatotroph-pituitary had the impact on the equal ability to produce GH among the offsprings coming from mother C, T3, and T5.
From the livestock maintenance method point of view, the concentration of offspring-rats GH from T2 and T4 mothers had a significant difference with the concentration of offspring GH from the mothers T3 and T5 (α<0.05). This difference was an indication that the nutritional components bone marrow from Donggala and Bali cattle with semi-intensive livestock practice had better nutritional potential in supporting fetal somatotroph-pituitary cell proliferation compared to bone marrow from Donggala and Bali cattle with traditional livestock practice. According to the opinion of previous researchers, the method of raising cattle will affect the nutritional composition of livestock products [16, 17]. Referring to Figure 1 and Table 1, when the offspring-rat was sixty days of age, the growth hormone production of offspring-rat from the C, T1, T2, T3, T4 and T5 mother, was decreasing. The decrease was related to the final growth and maturation phase of the organs like heart, liver, kidney, pancreas, brain or the linear growth and, the rat puberty phase. The ANOVA analysis of GH-concentration after sixty-day old indicated that there was a significant difference on the concentration of growth hormone on offspring-rat from the mother, which was given normal feeding, intervented by IUGR feed, intervened by the bone marrow substitution of feed at the α=0.001 (p<0.05).
4. Discussion
Based on Figure 1, the order of the concentration of GH of thirty-day old rat-offspring from the lowest to the highest is as follows: T1
According to Mirlesse et al. [18], growth retardation will occur if IGF-1 levels are low or placental GH production decreases. Referring to this opinion, somatotroph-pituitary retardation has occurred since pre-implantation or early intervention until birth. Furthermore, the non-optimal proliferation affects the maturity of the pituitary gland in producing GH to be not optimal. Different things were found in rats from mothers, which fed by normal feed and those, which were intervened by marrow-substituted feed during the pregnancy, where both groups of rats were able to produce the same GH concentration. Figure 1 clearly shows that the rat-offspring from the mother, which was given the normal feed or intervened by bone marrow-substitute feed, has higher growth hormone concentration compared to the rat-offspring from IUGR mother. Higher growth hormone production on rat-offspring from T2, T3, T4, and T5 mother indicates that the nutritional component of bone marrow semi-intensive and traditional of Donggala and Bali cattle is potential to support the somatotropic-pituitary growth.
After sixty-day old, the optimal production of growth hormone could still be found on rat-offspring from mother T2, T3, T4, and T5. It indicates that the nutritional component ability of bone marrow, that was substituted into the feed, were able to maintain somatotropic-pituitary quality. The nutrition like fatty or amino acids of marrow has a significant role in supporting somatotroph proliferation at the beginning of life to postnatal. A better maturity of the pituitary gland, from the rat-offspring that was given marrow-substituted feed, caused it to be able to produce higher GH compared to rats that were intervened by IUGR feed. Although there was a decrease in the growth hormone on sixty-day old rat-offspring as shown in Figure 1, the lowest concentration was still found on the rat-offspring whose mother intervened by IUGR feed (8.7 ng/ml).
The growth hormone, which was still produced, is needed for the process of maturing the sexual and reproductive organs. According to some of the results of previous studies, growth hormone deficiency or the occurrence of growth hormone resistance would cause retardation or cause a person not experiencing puberty [19]. Then in a cohort study conducted on sixty women who lacked growth hormone, it was found that only sixteen people experienced normal puberty, then ten people experienced retardation, and others did not occur puberty [8]. Delayed puberty also occurred in most rats that experienced growth hormone receptors knockout [20]. The important role of growth hormone in sexual maturation and reproductive organs is seen in the abduction of exogenous growth hormone which can accelerate the sexual maturation of monkeys [21], while, in children who have a growth hormone deficiency, exogenous adduction of GH will accelerate sexual maturation [21]. According to the references described above, the GH has a role since pre-implantation to the puberty phase. Afterwards, we will explain how the nutrients contained in the four types of marrow substitution contribute to the proliferation of somatotroph-pituitary, which will be explained as illustrated in Figure 2.
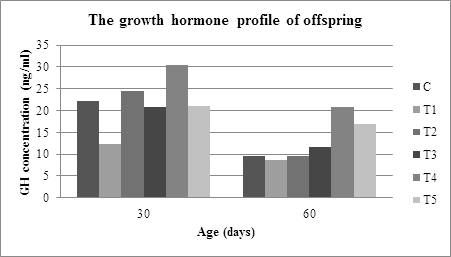
Figure 1: The profile of growth hormone of offspring on age thirty and sixty days.
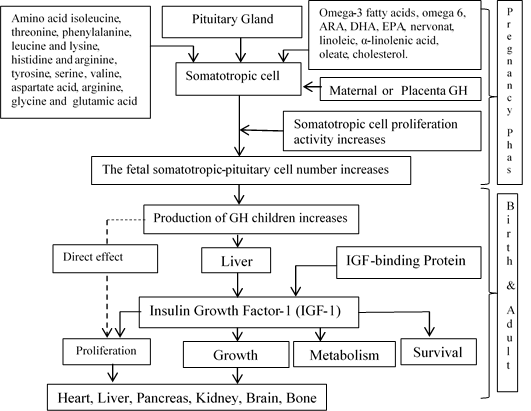
Figure 2: The hypothesis chart of the nutrition effect local beef bone marrow substituted on feed and it is the ability to stimulation increasing fetal pituitary-somatotropic cell proliferation, and so that happened increasing GH production.
Referring to the intervention done by this research, since the beginning of the pregnancy to the birth, it was found that fatty and amino acids integrated in the marrow-substituted feed would contribute towards cell proliferation since pre-implantation to the baby birth. Essential and non-essential fatty and amino acids will support cell proliferation through a direct or indirect mechanism. In direct mechanism, marrow fatty acids can be used for cell and organelle membranes synthesis. While, in indirect mechanism, this molecule converted into other molecules to support cell proliferation. In line with the things mentioned above, experts provide recommendations and opinions; fatty acids should be the key component in the pregnancy diet. If we perceive it from a physiological point of view, the long-chain polyunsaturated fatty acids (LC-PUFA) have an essential role in maternal-fetal metabolism [22]. Cohort studies found that higher intake of saturated fatty acids (SFA) and omega-3 at the beginning of pregnancy was associated with an increase in birth weight and small gestational age (SGA) on the population in South India [23].
Omega-3 are fatty acids that are very needed during cell proliferation, especially for cell membrane synthesis. According to Lazzarin et al. [24], ω-3 fatty acids are found in many parts of the body, one of which is a major component of cell membranes. The same opinion was also expressed by Krauss-Deutschmann et al. [25]. He stated that DHA is a key component of all cell membranes, but is found abundantly in the brain and retina.
Dunstan et al. [26] argue that EPA and DHA are essential for the proper fetal development and for maintaining health in old age. The longer fatty acids with double bonds will increase cell membrane fluidity; optimize cell signaling, and protein regulation [27]. Swanson et al. [28] expressed the same opinion, Omega-3 fatty acids, including EPA and DHA, are essential for the proper fetal development. Referring to some of the studies mentioned above, we believe that the component of omega-3 (including DHA and EPA) taken from the Donggala and Bali cattle’s bone marrow (with concentrations 0.45-0.53 g) are fatty acids that contribute to the proliferation of somatotropic cell of the pituitary.
Omega-6 fatty acids also have an important role in the process of pregnancy, one of which is regulating prostaglandin production, which, according to Kassem et al. [29] Omega-6 levels can control the synthesis of uterus PGF2α stimulated by n-3 fatty acids. Together with omega-3, these fatty acids are known to be responsible for placental weight, the results of the study show that deficiencies of these fatty acids can cause a decrease in placental and fetal mass [30]. The role of ALA, LA, DHA, ARA and the other derivatives of omega-3 or omega-6 contained in the mother's diet to the metabolism that occurs between mother and fetus are well understood. The inadequate intake of fatty acids during pregnancy can cause premature birth or the occurrence of intrauterine growth retardation (IUGR) [31].
In every 100 grams of local beef bone marrow contains the omega-6 between 1.16-1.30 grams, arachidonic acid between 33-52 mg, the α-linolenic acid between 0.53-0.62 grams, and linoleic acid between 1.09-1.22 grams. The collaboration among these fatty acids in supporting cell proliferation will have implications for the effectiveness of the process and produce better gland maturity in producing GH.
Oleate is one of the fatty acids found with quite high concentration in the marrow (between 23.67-34.69% of marrow). The results of previous studies have shown that these fatty acids have the potential as linoleic acid precursors [32]. It means that if the conversion of oleic on the marrow to linoleic occurs through the metabolic processes during pregnancy, then it will increase the concentration of linoleic. The linoleic acid result on this process can be used for the synthesis of membranes and the synthesis of other important molecules during growth.
The total cholesterol concentration of four types of marrow in this study was between 0.46-0.77%. The results of previous studies stated that cholesterol is a molecule that plays a role in determining the physical properties of the bilayers lipid membrane, such as fluidity and membrane elasticity [33]. Marrow cholesterol, which was integrated into the intervention feed during the pregnancy, will contribute to the proliferation through the optimization of cell membranes synthesis for its fluidity and elasticity. The amino acid components contained in the marrow are isoleucine, histidine, tyrosine, threonine, serine, valine, phenylalanine, proline, aspartate acid, alanine, arginine, leucine, lysine, glycine and glutamate acid with concentrations between 556.67-6838.86 ppm. These amino acids is possible to contribute to protein synthesis or peptides, which act as message molecules, structural proteins, enzymes, and hormones during the fetal growth.
Arginine is one of the amino acids that have a significant contribution to fetal growth. Zeitoun [34] reported that a combination of arginine-propylene glycol for eight weeks of pregnancy is not only increasing the growth and life sustainability of the fetal but also improving corpus luteum function. Arginine was a precursor of nitrogen for the synthesis of nitric oxide (NO), which acts as the primary signaling agent on almost all cells [35]. Polyamine and NO synthesized from arginine are molecules that are essential for placental growth and the process of angiogenesis (synthesis of new blood vessels) so that it indirectly affects the increase of blood flow to the uterine and fetal placenta [36]. The mechanism that is responsible for the adverse effects of high protein supplementation is not yet known, but rational protein supplementation will have an impact on the increase of intrauterine growth and reduce the incidence of premature births as is often found in cases of IUGR [37].
5. Conclusion
The semi-intensive and traditional Donggala and Bali cattle’s marrow-substituted feed formulation were able to increase the somatotroph-pituitary proliferation of the fetal if it was perceived from the production of thirty and sixty-day old rat-offsprings growth hormone.
References
- Murphy VE, Smith R, Giles WB, et al. Endocrine regulation of human fetal growth?: The role of the mother, placenta, and fetus. Endocr Rev 27 (2006): 141-169.
- Osmond C and Barker DJP. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect 108 (2000): 545-553.
- Yi S, Bernat B, Pal G, et al. Functional promiscuity of squirrel monkey growth hormone receptor toward both primate and nonprimate growth hormones. Mol Biol Evol 19 (2002): 1083-1092.
- Murray PG and Clayton PE. Endocrine control of growth. Am J Med Genet 163 (2013): 76-85.
- Lobiego PE, Wood TJJ, Chenn CM, et al. Nuclear translocation and anchorage of the growth hormone receptor. J Biol Chem 269 (1994): 31735-31746.
- Zachmann M. Interrelations between growth hormone and sex hormones: Physiology and therapeutic consequences. Horm Res Paediat 38 (1992): 1-8.
- Alsat E, Guibourdenche J, Luton D, et al. Human placental growth hormone. Am J Obstet Gynecol 177 (1997): 1526-1534.
- De Boer AM, Schoemaker J and van der Veen AE. Impaired reproductive function in women treated for growth hormone deficiency during childhood. J Clin Endocr 46 (1997): 681-689.
- Resnick CE, Adashi EY, D’Ercole AJ, et al. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev 6 (1985): 400-420.
- Kobayashi J, Mizunuma H, Kikuchi N, et al. Morphological assessment of the effect of growth hormone on preantral follicles from 11-day-old mice in an in vitro culture system. Biochem Biophys Res Commun 268 (2000): 36-41.
- Lucy MC, Thatcher WW, Collier RJ, et al. Effects of somatotropin on the conceptus, uterus, and ovary during maternal recognition of pregnancy in cattle. Domest Anim Endocrinol 12 (1995): 73-82.
- Advis JP, Ojeda SR and White SS. Activation of growth hormone short loop negative feedback delays puberty in the female ra. Endocrinology 108 (1981): 1343-1352.
- Mendoza C, Cremades N, Ruiz-Requena E, et al. Relationship between fertilization results after intracytoplasmic sperm injection, and intrafollicular steroid, pituitary hormone and cytokine concentrations. Hum Reprod 14 (1999): 628-635.
- Ovesen P, Ingerslev HJ, Orskov H, et al. Effect of growth hormone on steroidogenesis, insulin-like growth factor-I (IGF-I) and IGF-binding protein-1 production and DNA synthesis in cultured human luteinized granulosa cells. Eur J Endocrinol 140 (1994): 313-319.
- Tangkas IM, Sulaeman A, Anwar F, et al. Multinutrients from local cattle bone marrow in Central Sulawesi of Indonesia have the potential to improve the successful pregnancy rate and prevent slowing of fetal kidney growth. Pak J Nutr 18 (2019): 961-968.
- Wood JD, Enser M, Fisher AV, et al. Fat deposition, fatty acid composition and meat quality: A review. Meat Science 78 (2008): 343-358.
- Garcia PT, Pensel NA, Sancho AM, et al. Meat beef lipids in relation to animal breed and nutrition in Argentina. Meat Science 79 (2008): 500-508.
- Mirlesse V, Frankenne F, Alsat E, et al. Placental growth hormone levels in normal pregnancy and in pregnancies with intrauterine growth retardation. Pediatr Res 34 (1993): 439-442.
- Hull KL and Harvey S. Growth hormone: Roles in female reproduction. J Endocrinol 168 (2001): 1-23.
- Rudman CG, Tanner JM, Wilson ME, et al. Effects of growth hormone on the tempo of sexual maturation in female rhesus monkeys. J Clin Endocrinol Metab 68 (1989): 29-38.
- Stanhope R, Albanese A, Hindmarsh P, et al. The effects of growth hormone therapy on spontaneous sexual development. Horm Res Paediatr 38 (1992): 9-13.
- Koletzko B, Lien E, Agostoni C, et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 36 (2008): 5-14.
- Mani I, Dwarkanath P, Thomas T, et al. Maternal fat and fatty acid intake and birth outcomes in a South Indian population. Int J Epidemiol 45 (2016): 523-531.
- Lazzarin N, Vaquero E, Exacoustos C, et al. Low-dose aspirin and omega-3 fatty acids improve uterine artery blood flow velocity in women with recurrent miscarriage due to impaired uterine perfusion. Fertil Steril 92 (2009): 296-300.
- Krauss-etschmann S, Shadid R, Campoy C, et al. Effects of fish-oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter. Am J Clin Nutr 85 (2007): 1392-1400.
- Dunstan JA, Mitoulas LR, Dixon G, et al. The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: a randomized controlled trial. Pediatr Res 62 (2007): 689-694.
- Holthuis JCM and Menon AK. Lipid landscapes and pipelines in membrane homeostasis. Nature 510 (2014): 48-57.
- Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 510 (2012): 1-7.
- Kassem AA, Bakar ZA, Meng GY, et al. Effect of dietary n-6 to n-3 polyunsaturated fatty acid ratio on prostaglandin plasma levels and genes expression peroxisome proliferator-activated receptor (PPAR) in pregnant Sprague Dawley rats. Afr J Biotechnol 10 (2011): 8703-8708.
- Etin I, Giovannini Nc, Alvino G, et al. Intrauterine growth restriction is associated with changes in polyunsaturated fatty acid fetal-maternal relationships. Pediatr Res 52 (2002): 750-755.
- Yuan C and Bloch K. Conversion of oleic acid to linoleic acid. J Biol Chem 236 (1961): 1277-1279.
- Xu X and London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry 39 (2000): 843-849.
- Needham D and Nunn RS. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J 58 (1990): 997-1009.
- Zeitoun MM. L-arginine or glucose dependence required for fetal growth, survival and maternal progesterone during late pregnancy in ewes. Int J Anim Res 1 (2017): 1-9.
- Shi Jobgen W, Fried S, Fu W, et al. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17 (2006): 571-588.
- Wu G, Bazer F, Datta S, et al. Intrauterine growth retardation in livestock: Implications, mechanisms and solutions. Arch Tierzucht 51 (2008): 4-10.
- Brown LD, Green AS, Limesand SW, et al. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci 3 (2011): 428-444.
