The Distribution and Prognostic Significance of Tumour Infiltrating Lymphocytes in Invasive Breast Carcinoma of Egyptian Patients
Article Information
Amera M Sheha1, Fatma Badary2, Tarek Mohamed Elsaba1*, Michael S Toss1,3
1Department of Histopathology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
2Department of Histopathology, School of Medicine, Assiut University, Assiut, Egypt
3Division of Cancer and Stem Cell, School of Medicine, The University of Nottingham, Nottingham, United Kingdom
*Corresponding author: Dr. Tarek Elsaba, Associate professor, Department of Histopathology, South Egypt Cancer Institute, Assiut University, Egypt
Received: 16 July 2020; Accepted: 27 July 2020; Published: 24 August 2020
Citation:
Amera M Sheha, Fatma Badary, Tarek Mohamed Elsaba, Michael S Toss. The Distribution and Prognostic Significance of Tumour Infiltrating Lymphocytes in Invasive Breast Carcinoma of Egyptian Patients. Journal of Women’s Health and Development 3 (2020): 330-346.
View / Download Pdf Share at FacebookAbstract
Background: The relationship between tumour infiltrating lymphocytes (TILs) and racial background in invasive breast carcinoma (IBC) is not well studied. In this study, we assessed the association between TILs and various clinicopathological parameters and outcome in a cohort of Egyptian IBC patients.
Methods: This study included a retrospective cohort (n=184) of IBC diagnosed in South Egypt Cancer Institute. All haematoxylin and eosin (H&E) histological sections were retrieved and the percentage of stromal TILs was assessed by 3 pathologists. The interobserver concordance was assessed and the association between TILs and clinicopathological factors and patients’ outcome was analysed.
Results: There was an excellent interobserver concordance for TILs assessment (Inter-cluster correlation coefficient ICC=0.9). 70% of the cohort showed TILs density >10%. TILs score was higher in patients aged less than 50 years (p=0.004), higher tumour grade (p=0.001), no special type and special types of breast carcinoma (p=0.001), and hormonal receptor negativity (p=0.001).Triple negative breast cancer (TNBC) and HER2 enriched molecular subtypes showed the highest TILs density among the molecular classes (p=0.018).There was no significant association between TILs and outcome.
Conclusion: Our study revealed that TILs are associated with features of high-risk IBC. Although the distribution of TILs among various IBC characteristics in Egyptian patients was similar to other ethnicities, there was no association between TILs density and patient outcome. Further epidemiological and comparative studies are warranted.
Keywords
TILs, Breast cancer, Egyptian, Interobserver agreement, Prognostic
TILs articles TILs Research articles TILs review articles TILs PubMed articles TILs PubMed Central articles TILs 2023 articles TILs 2024 articles TILs Scopus articles TILs impact factor journals TILs Scopus journals TILs PubMed journals TILs medical journals TILs free journals TILs best journals TILs top journals TILs free medical journals TILs famous journals TILs Google Scholar indexed journals Breast cancer articles Breast cancer Research articles Breast cancer review articles Breast cancer PubMed articles Breast cancer PubMed Central articles Breast cancer 2023 articles Breast cancer 2024 articles Breast cancer Scopus articles Breast cancer impact factor journals Breast cancer Scopus journals Breast cancer PubMed journals Breast cancer medical journals Breast cancer free journals Breast cancer best journals Breast cancer top journals Breast cancer free medical journals Breast cancer famous journals Breast cancer Google Scholar indexed journals Egyptian articles Egyptian Research articles Egyptian review articles Egyptian PubMed articles Egyptian PubMed Central articles Egyptian 2023 articles Egyptian 2024 articles Egyptian Scopus articles Egyptian impact factor journals Egyptian Scopus journals Egyptian PubMed journals Egyptian medical journals Egyptian free journals Egyptian best journals Egyptian top journals Egyptian free medical journals Egyptian famous journals Egyptian Google Scholar indexed journals Interobserver agreement articles Interobserver agreement Research articles Interobserver agreement review articles Interobserver agreement PubMed articles Interobserver agreement PubMed Central articles Interobserver agreement 2023 articles Interobserver agreement 2024 articles Interobserver agreement Scopus articles Interobserver agreement impact factor journals Interobserver agreement Scopus journals Interobserver agreement PubMed journals Interobserver agreement medical journals Interobserver agreement free journals Interobserver agreement best journals Interobserver agreement top journals Interobserver agreement free medical journals Interobserver agreement famous journals Interobserver agreement Google Scholar indexed journals Prognostic articles Prognostic Research articles Prognostic review articles Prognostic PubMed articles Prognostic PubMed Central articles Prognostic 2023 articles Prognostic 2024 articles Prognostic Scopus articles Prognostic impact factor journals Prognostic Scopus journals Prognostic PubMed journals Prognostic medical journals Prognostic free journals Prognostic best journals Prognostic top journals Prognostic free medical journals Prognostic famous journals Prognostic Google Scholar indexed journals tumour articles tumour Research articles tumour review articles tumour PubMed articles tumour PubMed Central articles tumour 2023 articles tumour 2024 articles tumour Scopus articles tumour impact factor journals tumour Scopus journals tumour PubMed journals tumour medical journals tumour free journals tumour best journals tumour top journals tumour free medical journals tumour famous journals tumour Google Scholar indexed journals Immune-Oncology articles Immune-Oncology Research articles Immune-Oncology review articles Immune-Oncology PubMed articles Immune-Oncology PubMed Central articles Immune-Oncology 2023 articles Immune-Oncology 2024 articles Immune-Oncology Scopus articles Immune-Oncology impact factor journals Immune-Oncology Scopus journals Immune-Oncology PubMed journals Immune-Oncology medical journals Immune-Oncology free journals Immune-Oncology best journals Immune-Oncology top journals Immune-Oncology free medical journals Immune-Oncology famous journals Immune-Oncology Google Scholar indexed journals histopathology articles histopathology Research articles histopathology review articles histopathology PubMed articles histopathology PubMed Central articles histopathology 2023 articles histopathology 2024 articles histopathology Scopus articles histopathology impact factor journals histopathology Scopus journals histopathology PubMed journals histopathology medical journals histopathology free journals histopathology best journals histopathology top journals histopathology free medical journals histopathology famous journals histopathology Google Scholar indexed journals carcinoma articles carcinoma Research articles carcinoma review articles carcinoma PubMed articles carcinoma PubMed Central articles carcinoma 2023 articles carcinoma 2024 articles carcinoma Scopus articles carcinoma impact factor journals carcinoma Scopus journals carcinoma PubMed journals carcinoma medical journals carcinoma free journals carcinoma best journals carcinoma top journals carcinoma free medical journals carcinoma famous journals carcinoma Google Scholar indexed journals heterogeneously articles heterogeneously Research articles heterogeneously review articles heterogeneously PubMed articles heterogeneously PubMed Central articles heterogeneously 2023 articles heterogeneously 2024 articles heterogeneously Scopus articles heterogeneously impact factor journals heterogeneously Scopus journals heterogeneously PubMed journals heterogeneously medical journals heterogeneously free journals heterogeneously best journals heterogeneously top journals heterogeneously free medical journals heterogeneously famous journals heterogeneously Google Scholar indexed journals
Article Details
1. Introduction
Invasive breast cancer (IBC) is the most common cancer diagnosed in women worldwide and it is the second leading cause of cancer related deaths in females [1]. In Egypt, IBC represents 20% of all primary malignant tumours and ranks the first most common malignancy in females [2]. The choice of systemic therapy for IBC depends on various clinical and pathological parameters as including tumour histological type, grade, hormonal status, HER2 overexpression and proliferation index. Although these factors are vital for patient management, they ignore the role of the surrounding tumour microenvironment especially the immune response, which is critical for tumour progression, invasion and metastases [3]. The tumour immune microenvironment have been shown to modulate therapy response and prognosis, specifically in triple negative breast cancer (TNBC) and human epidermal growth factor receptor 2 (HER2) enriched subtypes, standing for a novel field of translational research in the era of effective immunotherapies [4]. The role of the host immune system in getting rid of malignant cells has been consolidated in the immunoediting theory [5]. Evasion of the host immunity is one of the main hallmarks of carcinogenesis [6]. The International Immune-Oncology Working Group published consensus guidelines for evaluating tumour infiltrating lymphocytes (TILs) using routinely stained haematoxylin and eosin (H&E) sections in IBC. Multiple systemic reviews and meta-analysis have reported the role of TILs in IBC [7, 8]. Moreover, in the recent edition of World Health Organisation (WHO) breast tumours classification, they recommended assessment of TILs in routine practice and introduced the term lymphocyte rich carcinoma as a subtype of IBC of no special type (NST) [9]. Although the role of TILs in IBC prognosis is undeniable, studies assessed the role of TILs among different ethnic and racial backgrounds, to the best of our knowledge, are limited. Thus, in this study we hypothesised that TILs density among Egyptian patients with IBC is unique. We retrospectively evaluated TILs in a cohort of IBC diagnosed and managed in Egypt and investigated its association with different clinicopathological characteristics and patients’ outcome.
2. Materials and Methods
2.1 Study cohort
This retrospective study included a cohort of primary IBC (n=184), diagnosed and managed at South Egypt Cancer
Institute, Assiut, Egypt from 2014 to 2016. All available clinicopathological data including patient age at diagnosis, tumour grade, histological subtype, tumour size, lymph node status, lymphovascular invasion (LVI), presence of necrosis, associated ductal carcinoma in situ (DCIS), biomarker defined classes based on both oestrogen receptor (ER) and HER2 status (defined as ER positive/HER2 negative, ER positive/HER2 positive, ER negative/HER2 positive and ER negative/HER2 negative) [9] were collected. In addition, all cases were classified into molecular subtypes according to the immunohistochemical evaluation of ER, progesterone receptor (PR) and HER2 expression into luminal, HER2 enriched and TNBC subtypes. Tumour grade was evaluated according to Nottingham grading system [10], while tumour stage was described using American Joint Committee on Cancer Staging [11]. All cases included in this study were treated primarily by surgery followed by adjuvant therapy either hormonal, chemotherapy or both. Chemotherapy regimen was offered according to the institutional protocol which was mainly CMF for 6 cycles. Patients treated with neoadjuvant therapy were excluded. Outcome data was retrieved in terms of local recurrence free interval (LRFI) (defined as time in months between diagnosis and occurrence of ipsilateral tumour recurrence), distant metastases free interval (DMFI) (defined as time in months between diagnosis and occurrence of DM) and breast cancer specific survival (BCSS) (defined as time in months between diagnosis and patient’s last date known to be alive or death from breast cancer). With a median follow up period of 32 months, there were 4 cases (2%) with local recurrence, 16 cases with distant metastases (9%) and 11 cases (6%) deceased from breast cancer.
2.2 Histological evaluation and TILs scoring
All available H&E stained sections from the selected cases were retrieved from histopathology department archive and reviewed for section quality and suitability for TILs evaluation. Moreover, all cases were reclassified according to the recent WHO classification of breast tumours [9, 12]. Stromal TILs have been assessed in H&E stained sections following the international working group recommendations [13]. Briefly, one representative section for each case was selected that showed at least 30%viable invasive tumour tissue. Only TILs within the border of invasive tumour were evaluated. TILs surrounding areas of necrosis, artefacts or extensive fibrosis were not included in the scoring. The average percentage of stromal TILs was reported. In addition, cases with TILs distributed mostly (>95%) at the invasive margin were recorded. The invasive tumour margin was defined as a narrow area at the tumour/host interface with a width of approximately 1mm between the invasive edge of carcinoma tissue and the adjacent non-tumorous fibro-adipose stroma of the breast tissue [14]. Lymphoid aggregates at the invasive margin were recorded if present. Scoring of all cases has been performed by 3 pathologists blinded to patient data to evaluate the inter-observer reliability. The average score for each case was obtained as final scores for purpose of analysis with clinicopathological data and outcome. For the purpose of analysis, TILs were categorised into 3 groups (low <10%, intermediate 11-50%, and high ≥50%) [3].
2.3 Statistical analysis
Statistical analysis wasperformed using SPSS version 20 for windows. The inter-rater reliability was assessed using inter-cluster correlation coefficient test (ICC). Mann Whitney U and Kruskal Wallis tests were used to evaluate the association between TILs as a continuous variable and clinicopathological parameters. Survival analysis was performed by log ranks tests and the Kaplan–Meier curves. Statistical significance was defined as p< 0.05. The study was approved by the institutional ethics committee number (17100297) under the title “prognostic significance of tumour infiltrating lymphocytes in invasive breast carcinoma”. All patients included were consented to participate in the study and to use their materials in research. All samples were pseudo-anonymised and stored in compliance with the Human Tissue Act. The study was performed in accordance with the Declaration of Helsinki.
3. Results
3.1 Clinicopathologic characteristics
Among cases included in study, there were 157 cases (85%) NST of type, 7 (4%) invasive lobular carcinoma (ILC) and 15 (8%) mixed NST and special type. Clinicopathological characteristics of the patients are shown in Table 1.
|
Clinicopathological variable |
Frequency (Number/Percentage) |
|
Age at diagnosis (Years) ≤ 50 > 50 |
77 (42%) 107 (58%) |
|
Tumour size (mm) <20 20-50 >50 |
7 (4%) 139 (75%) 38 (21%) |
|
Histological type Invasive carcinoma, No special type Invasive lobular carcinoma Mixed NST and special type Pure special type |
157 (85%) 7 (4%) 15 (8%) 5 (3%) |
|
Grade 1 2 3 |
3 (2%) 153 (83%) 28 (15%) |
|
Oestrogen receptor Negative Positive Not available |
57 (31 %) 113 (61 %) 14 (8 %) |
|
Progesterone receptor Negative Positive Not available |
67 (36 %) 101 (55 %) 16 (9 %) |
|
HER2status Negative Positive Not available |
82 (45 %) 33 (18 %) 69 (37 %) |
|
Molecular classification Luminal Her2 enriched TNBC Not available |
120 (65 %) 13 (7 %) 22 (12 %) 29 (16 %) |
|
Biomarker defined subtypes ER+ HER2+ ER+HER2- ER- HER2+ ER- HER2- Not available |
19 (10 %) 53 (29 %) 14 (8 %) 25 (13 %) 73 (40 %) |
|
Tumour Stage I II II IV Not available |
10 (5 %) 60 (33 %) 39 (21 %) 16 (9 %) 59 (32 %) |
|
Local recurrence Yes No Not available |
4 (2 %) 126 (69 %) 54 (29 %) |
|
Distant metastases Yes No Not available |
16 (9 %) 114 (62 %) 54 (29 %) |
|
State of the patient Died Alive Not available |
11 (6 %) 131 (71 %) 42 (23 %) |
|
Tumour infiltrating lymphocytes Mild Moderate Dense |
56 (30%) 117 (64%) 11 (6%) |
|
Tertiary lymphoid structures Present Absent |
8 (4 %) 176 (96 %) |
|
Pattern of TILs distribution Invasive margin Whole tumour |
66 (36 %) 118 (64 %) |
Table 1: Clinicopathological features of the study cohort.
3.2 TILs scoring concordance and distribution
The interobserver concordnace of TILs scoring between the three observers was excellent (ICC= 0.95; p<0.001). The cohort showed unimodal non parametric distribution of TILs. The median TILs density was 15% (range 0-80%). Low TILs density (<10%) was observed in 30% of the cohort, moderate (11-50%) in 64% and high TILs density (>50% of the stroma occupied by inflammatory cells) in 6% of the cases. Figures 1 and 2 show examples of various TILs scores. Lymphoid aggregate at the invasive tumour margin were observed in 8 cases (4%) (Figure 3). TILs were heterogeneously distributed in the whole tumour (invasive margin and tumour centre) in 118 cases (64 %) while 66 cases (36 %) showed >95% of TILs at the invasive margin (Figure 4).
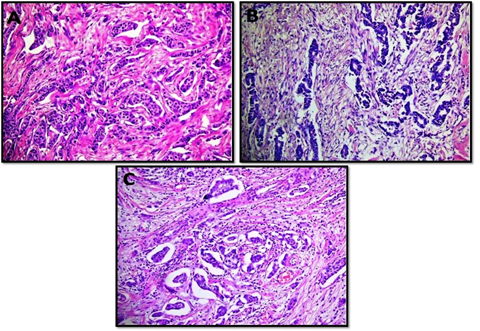
Figure 1: Microphotographic examples of invasive breast carcinomas with low (A and B) and intermediate (C) tumour infiltarting lymphocyte densities. All images at x20 magnifcation.
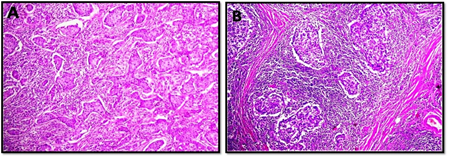
Figure 2: Microphotographic examples of invasive breast carcinomas with high tumour infiltrating lymphocyte densities. All images at x10 maginfcation.
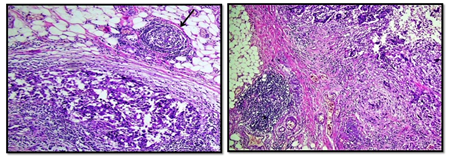
Figure 3: Examples of tertiary lymphoid structure, located at the invasive margin of breast carcinoma case (x10 magnification).
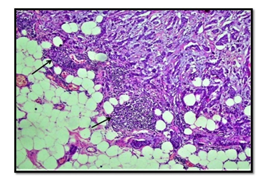
Figure 4: Example of invasive breast carcinoma showing high immune cell density at the invasive margin (arrow) and near complete absence at tumour centre. (x10 magnification).
3.3 Association of TILs and clinicopathological characteristics
The median score of TILs was higher in younger age patients <50 years (p=0.004). TILs were denser in larger (>20mm) than smaller tumours (p=0.01). There was statistically significant difference between the TILs density among different histological types (p=0.001) where the highest median TILs score was present in NST type while ILC showed the least density. There is a steady increase of TILs density from grade 1 to grade3tumours. Median TILs score was 5, 10and 27.5 % in grade 1, 2 and 3, respectively (p=0.001). TILs were more frequent in cases with hormonal receptors negativity (p=0.001 for both ER and PR). There was no association between TILs and HER2 overexpression (p=0.308). Among molecular BC subtypes, TILs were statistically significant associated with TNBC and HER2 enriched subtypes (p=0.018). Table 2 summarise the association of TILs with different clinicopathological characteristics. No statistically significant association was detected between average stromal TILs and lymphoid aggregates or patterns of TILs distribution. Moreover, neither lymphoid aggregates nor patterns of TILs distribution were associated with any other clinicopathological characters.
3.4 The prognostic significance of TILs
At univariate analysis, tumour stage, age at diagnosis and molecular subtypes showed association with BCSS (Table 3). At multivariate analysis, tumour stage was independent prognostic factor for overall survival. There was no significant association between TILs and LRFI (p=0.453), DMFI (p=0.342) nor BCSS (p=0.447); (Figure 5). In TNBC group; two of12 cases (17%) showed local recurrence with TILs level between 11-50% while no events occurred in other two TILs groups. Association of TILs with DM revealed that one patient, 2and nil showed DM in cases with TILs score<10%, 11-50% and >50%, respectively however it did not reach statistical significance
(p=0.601); (Figure 6).
|
Characteristics |
Median TILs score (%) |
p-value |
|
Age at diagnosis ≤50 years (N = 77) >50 years (N = 107) |
20.00 10.00 |
0.004 |
|
Tumour size <20 mm (N = 7) 20-50 mm (N = 139) >50 mm (N = 38) |
10.00 15.00 10.00 |
0.010 |
|
Histological type Invasive carcinoma, NST (N = 157) Invasive lobular carcinoma (N = 7) Mixed NST and special type (N = 15) Pure special type (N = 5) |
15.00 5.00 5.00 37.50 |
0.001 |
|
Tumour grade Grade 1 (N = 3) Grade 2 (N = 153) Grade 3 (N = 28) |
5.00 10.00 27.50 |
0.001 |
|
Lymphovascular invasion Present (N = 135) Absent (N = 49) |
10.00 15.00 |
0.511 |
|
Ductal carcinoma in situ Present (N = 81) Absent (N = 103) |
15.00 10.00 |
0.661 |
|
Tumour necrosis Present (N = 62) Absent (N = 122) |
20.00 10.00 |
0.09 |
|
Oestrogen receptor status Negative (N = 57) Positive (N = 113) |
25.00 10.00 |
0.001 |
|
Progesterone receptor Negative (N = 67) Positive (N = 101) |
25.00 10.00 |
0.001 |
|
HER2 status Negative (N = 82) Positive (N = 33) |
15.00 20.00 |
0.308 |
|
Molecular classification Luminal (N = 120) Her2 enriched (N = 13) TNBC (N= 22) |
10.00 25.00 25.00 |
0.018 |
|
Biomarker defined subtypes ER+, HER2+ (N = 19) ER+, HER2- (N = 53) ER-, HER+ (N = 14) ER-, HER2- (N = 25) |
15.00 10.00 25.00 25.00 |
0.03 |
|
Tertiary lymphoid structure Present (N = 8) Absent (N = 176) |
12.50 15.00 |
0.595 |
|
Pattern of TILs distribution Whole tumour (N = 118) Invasive margin (N = 66) |
15.00 12.50 |
0.622 |
Significant p values are in Bold
Table 2: Association between tumour infiltrating lymphocytes and other clinicopathological features.
|
Variable |
BCSS |
LRFI |
DMFI |
|||
|
Log rank |
p value |
Log rank |
p value |
Log rank |
p value |
|
|
Stage |
13.6 |
0.003 |
14.1 |
0.003 |
175.6 |
0.001 |
|
Age at diagnosis |
4.09 |
0.04 |
1.03 |
0.308 |
0.2 |
0.649 |
|
Molecular classification |
14.9 |
0.001 |
5.8 |
0.054 |
0.27 |
0.871 |
|
Tumour infiltrating lymphocytes |
1.61 |
0.44 |
1.58 |
0.45 |
2.14 |
0.34 |
Significant p values are in Bold
Table 3: Univariate analysis of different clinicopathological factors and clinical outcome.
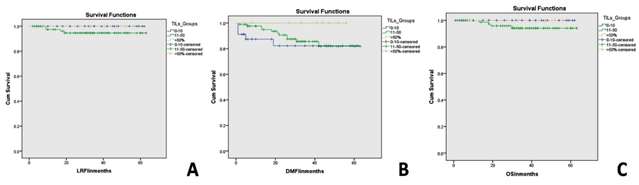
Figure 5: Kaplan Meier survival curves showing the association between tumour infiltrating lymphocytes density and local recurrence free interval (A), distant metastasis free interval (B) and breast cancer specific survival (C) in the whole study cohort.
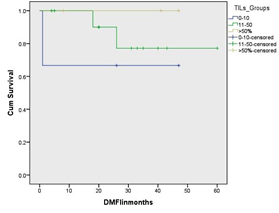
Figure 6: Kaplan Meier survival curves showing the association between tumour infiltrating lymphocytes density and distant metastasis free interval in triple negative breast cancer subtypes. Although it is not statistically significance, there is an obvious difference of the outcome between tumours with low TILs density and other groups.
In HER2 enriched subtype, TILs were not statistically significant associated with DM or BCSS(p=0.705, p=0.578, respectively).There was no prognostic significance of TILs distribution at the invasive margin in terms of LRFI (p=0.857), DMFI (p=0.684) and BCSS(p=0.784). No prognostic significance of lymphoid aggregates formation was observed.
4. Discussion
Tumour infiltrating lymphocytes (TILs) constitute a key component in tumour micro-environment that represent the local immune response against tumour [15]. Based on the emerging importance of TILs in IBC, a consensus guideline to standardise evaluation methodology of TILs assessment in H&E stained sections [13]. One of the main aims of publishing TILs evaluation methodology was to achieve robustness and reproducibility of the assessment method with achievement of good inter-observer agreement between pathologists. In this study, three pathologists have reviewed 184 BC cases and scored TILs as a continuous variable as recommended. Interestingly, scoring of TILs showed excellent interobserver agreement. This supports that the guidelines could consolidate the use of TILs in clinical practice. TILs represent an important prognostic and potentially predictive biomarker in many different solid tumour types such as melanoma [16], non-small cell lung cancer [17] and colorectal carcinoma [18]. A strong association between TILs, prognosis of IBC patients and treatment response has been reported in a number of retrospective series including about 17,000 patients [19, 20]. Difference in TILs value within various ethnicities was reported previously [21]. Despite the large prevalence of BC in Africa, there is a large gap in the data about pathologic features of BC. The prognosis of African patients is less favourable than their counterparts in higher income countries [22]. There is very limited information about TILs in African population which may be different from American and European populations; possibly due to differences in intrinsic tumour features, genetic background and carcinogen exposure [22]. So, better understanding about the prognostic biomarkers in African patients is essential. We retrospectively evaluated TILs in a cohort of IBC from Egypt with median follow up of 32 months. TILs scores were higher in patients <50 years, similar to the report from Tianet al. [23] who found that TILs scores were higher in younger patients in studies conducted on Chineese patients. The mechanism of relationship between TILs and age is questionable, however it may be explained byyounger patients with BC usually exhibit more aggressive features and more likely to be hormone receptor negative which have more antigenicity and hence more immune reaction [25].
Our results showed statistically significant association of TILs scores with different size groups. Also, median TILs score gradually decrease with higher stage. One study showed that high CD8+ TILs positively correlate with tumour size less than 50 mm [26]. This could be related to the antitumor effect of CD8+ TILs, so they control the growth of the tumour by balancing the anti-tumour and pro-tumour immune responses [26]. Association of TILs with nodal stage revealed that highest TILs scores were in patients without nodal metastases. The correlation between increased TILs and lymph node status was first reported in gastric cancer [27] and melanoma [28]. One previous study on European reported that increased number of TILs was associated with lower number of lymph nodes metastases, suggesting that tumours with higher TILs have less aggressive clinical course [29]. Regarding association of TILs with different histological types, we found that highest TILs scores were detected in NST carcinoma. Metaplastic carcinoma cases showed absence of hormone receptor expression which may contribute to high TILs amount in this rare special type [30]. Jonejaet al reported that PD-L1 expression is significantly higher in metaplastic carcinoma than in NST tumours, which contribute to poor clinical outcome [31]. We demonstrated that TILs are lowest in ILC and mixed invasive ductal carcinoma with special type. Similarly, Desmedtet al [32] reported that most ILCs have low TILs level compared with NST tumours in British population. ILCs are characterised by being mostly low grade and hormonal receptor positive tumours which may attribute to low lymphocytic response [32]. The association of TILs with newly defined biomarker types was statistically significant and not surprisingly, TILs were higher in ER negative tumours. The updated classification acknowledged these clinical relevant subtypes of invasive carcinoma as these subtypes are different in pathogenesis, treatment response and prognosis [9]. The overview acknowledges the treatment-relevant subtypes of invasive carcinoma (based on ER and HER2 status) and new data is added to support the differences in pathogenesis, treatment response and prognosis of these clinically relevant groupings.
In agreement with previous studies [23, 33-35], high TILs correlated with higher histological grade and ER negative tumours in our study. High grade carcinomas are commonly of TNBC [36] and HER2 enriched subtypes [37]. The frequency of high TIL levels depend on the molecular subtype of BC [38]. Our study showed that median TILs score was higher in TNBC and HER2 enriched subtypes than luminal subtype. TNBC is usually common among African patients than White and European populations. This was previously described by multiple studies which reported that the median percentage of TILs was highest in ER negative and HER2 negative tumours [38]. Moreover, Papaioannouet al.[15] reported that dense lymphocytic infiltrate was observed in HER2 enriched and triple negative patients. This could possibly be due to high somatic mutational load in those specific subtypes, leading to appearance of neo-antigens that stimulate the immunologic response [39]. Our study showed no significant prognostic association between TILs and patient outcome neither for the whole cohort or in TNBC subgroup. This result is in agreement with previous study done by Park et al who reported that evaluation of TILs may not be useful in predicting survival in TNBC, on a study included Korean patients [40]. Contrary to the results of our study, results of previous studies that included data of randomised clinical trials evaluating data of 16,000 patients with available clinical follow up data, suggested that TIL expression is associated with prognosis in TNBC patients (19, 41). These studies were conducted mainly on non-African populations; thus such ethnic differences and genetic backgrounds may contribute for this contradictory. Other factors include cohort sample size and difference in management and follow up protocols among various institutions.
TILs were not prognostic in HER2 enriched cases in our study. This is contrary to data from NeoALLTO study trial reporting relationship between recurrence free survival and TILs population in HER2 positive patients [42]. However, the use of antiHER2 (trastuzumab) adjuvant therapy complicate the interpretation of data for HER2 enriched tumours [43] Similar to our results, Loiet al. reported that TILs have no prognostic value in hormonal receptor positive breast tumours, although their study included a cohort with different ethnic background [38] . The short median follow-up period in our study may be the reason for the discrepancy in the prognostic value of TILs between our study and other studies. Another limitation in this study is the lack of defining various types of immune cells using immunohistochemistry for B and T lymphocytes and other inflammatory cells that were used in other studies [44, 45]. The results of the previous studies showed the prognostic value of immunohistochemical analysis of TILs as the different types of TILs surrounding tumour have different roles in tumour suppression or progression. For instance, the negative effects of FOXP3+TILs on survival have been demonstrated in TNBC [44]. Contrary, CD8 + TILs correlated with better survival [45]. Since TILs are heterogeneously distributed, we separately evaluated TILs at the invasive margin and reported presence or absence of tertiary lymphoid structure (TLS), in order to find any clinical implication of tumour heterogeneity. Cases with TILs distributed mostly at the invasive margin and cases with TLS did not show any statistically significant association with patient outcome in our study. In other cancers, for example colorectal cancer, dense inflammatory infiltrate at the invasive margin is a good prognostic factor [18]. Over the past years, multiple studies have attempted to examine the relationship between the inflammatory infiltrate at the invasive margin and survival in IBC. However, the results were conflicting[46].Recently, Romagnoliet al.[47]looked at quantity and distribution of various immune cell subtypes at invasive margin and tumour centre. They found no statistically significant association in quantity and distribution of TIL ratios and subsets. Moreover, no statistically significant difference was detected when comparing relapse with non-relapse group. Koing et al [14] reported that TILs infiltration pattern in primary BC differed in invasive margin and tumour centre significantly, adding evidence that the invasive tumour margin is an immunologically active area .Further studies are needed to understand the exact role and clinical significance of TILs density and different TILs populations in invasive margin. Of note, the retrospective design of this study is a limitation as it decreases the statistical impact of the study while other studies assessed TILs in prospective trials [38, 41, 48]. However, we believe that it may clarify interesting data about association of TILs with pathological and clinical characteristics in Egyptian cohort. TILs have the potential to act as surrogate marker for prognosis.
5. Conclusion
Our study revealed that TILs are higher in younger patients and in special types of breast carcinoma in Egyptian patients similar to other ethnic groups. Moreover, a meaningful correlation was observed with respect to association of TILs with high grade tumours. TILs are higher in TNBC and HER2 enriched subtypes. However, we did not find any prognostic value in our study which may reflect difference behaviours of TILs in IBC of Egyptian population. Various mechanistic and comparative studies involving large cohort of patients from different ethnic and genetic backgrounds are highly warranted to investigate the prognostic and therapeutic value of TILs in IBC.
References
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66 (2016): 271-289.
- Mokhtar N, Salama A, Badawy O, Khorshed E, Mohamed G, Ibrahim M, et al. Cancer pathology registry, A 12 year registry (2000-2011) (2016): 238.
- Hida AI, Sagara Y, Yotsumoto D, Kanemitsu S, Kawano J, Baba S, et al. Prognostic and predictive impacts of tumor-infiltrating lymphocytes differ between Triple-negative and HER2-positive breast cancers treated with standard systemic therapies. Breast Cancer Res Treat 158 (2016): 1-9.
- McIntire PJ, Zhong E, Patel A, Khani F, D'Alfonso TM, Chen Z, et al. Hotspot enumeration of CD8+ tumor-infiltrating lymphocytes using digital image analysis in triple-negative breast cancer yields consistent results. Hum Pathol 85 (2019): 27-32.
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 22 (2004): 329-360.
- Weinberg R, Hanahan D. The hallmarks of cancer. Cell 100 (2000): 57-70.
- Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen KJPo. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PLoS One 11 (2016): e0152500.
- Yu X, Zhang Z, Wang Z, Wu P, Qiu F, Huang JJC, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol 18 (2016): 497-506.
- Hoon Tan P, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, et al. The 2019 WHO classification of tumours of the breast. Histopathology (2020).
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19 (1991): 403-410.
- Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67 (2017): 93-99.
- Classification of Tumours Editorial Board. Breast tumours. 5th ed. Lyon (France): International Agency for Pesearch on Cancer (2019).
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group. Ann Oncol 26 (2014): 259-271.
- König L, Mairinger FD, Hoffmann O, Bittner A-K, Schmid KW, Kimmig R, et al. Dissimilar patterns of tumor-infiltrating immune cells at the invasive tumor front and tumor center are associated with response to neoadjuvant chemotherapy in primary breast cancer. BMC Cancer 19 (2019): 120.
- Papaioannou E, Sakellakis M, Melachrinou M, Tzoracoleftherakis E, Kalofonos H, Kourea EJAr. A Standardized Evaluation Method for FOXP3+ Tregs and CD8+ T-cells in Breast Carcinoma: Association With Breast Carcinoma Subtypes, Stage and Prognosis. Anticancer Res 39 (2019): 1217-1232.
- Antohe M, Nedelcu RI, Nichita L, Popp CG, Cioplea M, Brinzea A, et al. Tumor infiltrating lymphocytes: The regulator of melanoma evolution. Oncol Lett 17 (2019): 4155-4161.
- Raju S, Joseph R, Sehgal S. Review of checkpoint immunotherapy for the management of non-small cell lung cancer. ImmunoTargets Ther 7 (2018): 63-75.
- Klintrup K, Makinen JM, Kauppila S, Vare PO, Melkko J, Tuominen H, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer 41 (2005): 2645-2654.
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31 (2013): 860-867.
- Pruneri G, Gray KP, Vingiani A, Viale G, Curigliano G, Criscitiello C, et al. Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res Treat 158 (2016): 323-331.
- Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, Clark E, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. The Lancet Oncol 18 (2017): 52-62.
- Mremi A, Broadwater G, Jackson K, Amsi P, Mbulwa C, Hyslop T, et al. Breast cancer in Tanzanian, black American, and white American women: An assessment of prognostic and predictive features, including tumor infiltrating lymphocytes. PLoS One 14 (2019): e0224760.
- Tian T, Ruan M, Yang W, Shui R. Evaluation of the prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers. Oncotarget 7 (2016): 44395-44405.
- Zgura A, Galesa L, Bratila E, Anghel R. Relationship between Tumor Infiltrating Lymphocytes and Progression in Breast Cancer. Maedica 13 (2018): 317-320.
- Chen H-L, Zhou M-Q, Tian W, Meng K-X, He H-F. Effect of Age on Breast Cancer Patient Prognoses: A Population-Based Study Using the SEER 18 Database. PLoS One 11 (2016): e0165409-e.
- Titipungul T, Chaisuriya N, Waraasawapati S, Koonmee S, Sangkhamanon S. Predictive Relevance of Tumor-infiltrating Lymphocytes in Breast Cancer. Pathology 46 (2014): S60
- Kim JY, Kim CH, Lee Y, Lee JH, Chae Y-SJP. Tumour infiltrating lymphocytes are predictors of lymph node metastasis in early gastric cancers. Pathology 49 (2017): 589-595.
- Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 30 (2012): 2678-2683.
- Caziuc A, Schlanger D, Amarinei G, Dindelegan GC. Can Tumor-Infiltrating Lymphocytes (TILs) Be a Predictive Factor for Lymph Nodes Status in Both Early Stage and Locally Advanced Breast Cancer? J Clin Med 8 (2019): 545.
- Afkhami M, Schmolze D, Yost SE, Frankel PH, Dagis A, Amanam IU, et al. Mutation and immune profiling of metaplastic breast cancer: Correlation with survival. PLoS One 14(2019): e0224726.
- Joneja U, Vranic S, Swensen J, Feldman R, Chen W, Kimbrough J, et al. Comprehensive profiling of metaplastic breast carcinomas reveals frequent overexpression of programmed death-ligand 1. J Clin Pathol 70 (2017): 255-259.
- Desmedt C, Salgado R, Fornili M, Pruneri G, Van den Eynden G, Zoppoli G, et al. Immune Infiltration in Invasive Lobular Breast Cancer. J Nat Cancer Instit 110 (2018): 768-776.
- Kurozumi S, Matsumoto H, Kurosumi M, Inoue K, Fujii T, Horiguchi J, et al. Prognostic significance of tumour-infiltrating lymphocytes for oestrogen receptor-negative breast cancer without lymph node metastasis. Oncol Lett 17 (2019): 2647-2656.
- Sonderstrup IMH, Jensen MB, Ejlertsen B, Eriksen JO, Gerdes AM, Kruse TA, et al. Evaluation of tumor-infiltrating lymphocytes and association with prognosis in BRCA-mutated breast cancer. Acta Oncol 58 (2019): 363-370.
- Miyoshi Y, Shien T, Ogiya A, Ishida N, Yamazaki K, Horii R, et al. Associations in tumor infiltrating lymphocytes between clinicopathological factors and clinical outcomes in estrogen receptor-positive/human epidermal growth factor receptor type 2 negative breast cancer. Oncol Lett 17 (2019): 2177-2186.
- Geyer FC, Pareja F, Weigelt B, Rakha E, Ellis IO, Schnitt SJ, et al. The Spectrum of Triple-Negative Breast Disease: High- and Low-Grade Lesions. Am J Pathol 187 (2017): 2139-2151.
- Padmanabhan R, Kheraldine HS, Meskin N, Vranic S, Al Moustafa AE. Crosstalk between HER2 and PD-1/PD-L1 in Breast Cancer: From Clinical Applications to Mathematical Models. Cancers 12 (2020).
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31 (2013): 860-867.
- Pruneri G, Vingiani A, Bagnardi V, Rotmensz N, De Rose A, Palazzo A, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol 27 (2015): 249-256.
- Park HS, Heo I, Kim JY, Kim S, Nam S, Park S, et al. No effect of tumor-infiltrating lymphocytes (TILs) on prognosis in patients with early triple-negative breast cancer: Validation of recommendations by the International TILs Working Group 2014. J Surg Oncol 114 (2016): 17-21.
- Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25 (2014): 1544-1550.
- Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol 1 (2015): 448-455.
- Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nature Rev Clin Oncol 13 (2016): 228-241.
- Jiang D, Gao Z, Cai Z, Wang M, He J. Clinicopathological and prognostic significance of FOXP3+ tumor infiltrating lymphocytes in patients with breast cancer: a meta-analysis. BMC Cancer 15 (2015): 727.
- Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res 17 (2015): 124.
- Mohammed ZM, Going JJ, Edwards J, Elsberger B, Doughty JC, McMillan DC. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer 107 (5): 864-873.
- Romagnoli G, Wiedermann M, Hubner F, Wenners A, Mathiak M, Rocken C, et al. Morphological Evaluation of Tumor-Infiltrating Lymphocytes (TILs) to Investigate Invasive Breast Cancer Immunogenicity, Reveal Lymphocytic Networks and Help Relapse Prediction: A Retrospective Study. Int J Mol Sci 18 (2017).
- Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32 (2014): 2959.
