The Clinical Heterogenicity and the Diagnostic Challenges of Idiopathic Inflammatory Myopathies
Article Information
Fadi Hassan1, 2*, Amir Saab1, 2, Ziv Paz2, 3, Edith Abramovici2, 4, Mohammad E Naffaa2, 3
1Department of Internal Medicine, Galilee Medical Center, Nahariya, Israel
2Azrieli Faculty of Medicine, Bar-Ilan University, Sefat, Israel
3Rheumatology Unit, Galilee Medical Center, Nahariya, Israel
4Department of Radiology, Galilee Medical Center, Nahariya, Israel
*Corresponding Author: Fadi Hassan, Department of Internal Medicine E, Galilee Medical Center, Nahariya, Israel
Received: 29 February 2020; Accepted: 13 March 2020; Published: 20 March 2020
Citation:
Fadi Hassan, Amir Saab, Ziv Paz, Edith Abramovici, Mohammad E Naffaa. The Clinical Heterogenicity and the Diagnostic Challenges of Idiopathic Inflammatory Myopathies. Fortune Journal of Rheumatology 2 (2020): 032-041.
View / Download Pdf Share at FacebookAbstract
Objective: The idiopathic inflammatory myopathies (IIMs) are a heterogeneous group of disorders. Determining the diagnosis of the specific type of IIM based on the current diagnostic criteria correlate poorly with the clinical course and outcomes. New and more accurate classification criteria are needed.
Methods: Five cases of IIM who were followed at the rheumatology division at Galilee Medical Center between 2017 and 2019. The data was retrospectively extracted from the hospital electronical medical record
Results: Records of 5 patients diagnosed with IIMs were reviewed including 3 patients diagnosed with Antisynthetase syndrome (AAS) and 2 patients diagnosed with Dermatomyositis (DM) and Polymoysitis (PM) respectively. The clinical presentation of those patients was heterogeneous as well as their response to treatment.
Conclusions: The current classification criteria aims to classify the patients into homogenous groups. However, even with the current classification criteria, patients diagnosed within the same subgroup, e.g. AAS, may still have variable clinical presentations and unpredictable response to treatment, emphasizing the unmet need for a better classification criteria.
Keywords
Idiopathic inflammatory myopathies, Heterogenicity, Challenges, Biologic treatments
Idiopathic inflammatory myopathies articles, Heterogenicity articles, Challenges articles, Biologic treatments articles
Idiopathic inflammatory myopathies articles Idiopathic inflammatory myopathies Research articles Idiopathic inflammatory myopathies review articles Idiopathic inflammatory myopathies PubMed articles Idiopathic inflammatory myopathies PubMed Central articles Idiopathic inflammatory myopathies 2023 articles Idiopathic inflammatory myopathies 2024 articles Idiopathic inflammatory myopathies Scopus articles Idiopathic inflammatory myopathies impact factor journals Idiopathic inflammatory myopathies Scopus journals Idiopathic inflammatory myopathies PubMed journals Idiopathic inflammatory myopathies medical journals Idiopathic inflammatory myopathies free journals Idiopathic inflammatory myopathies best journals Idiopathic inflammatory myopathies top journals Idiopathic inflammatory myopathies free medical journals Idiopathic inflammatory myopathies famous journals Idiopathic inflammatory myopathies Google Scholar indexed journals Heterogenicity articles Heterogenicity Research articles Heterogenicity review articles Heterogenicity PubMed articles Heterogenicity PubMed Central articles Heterogenicity 2023 articles Heterogenicity 2024 articles Heterogenicity Scopus articles Heterogenicity impact factor journals Heterogenicity Scopus journals Heterogenicity PubMed journals Heterogenicity medical journals Heterogenicity free journals Heterogenicity best journals Heterogenicity top journals Heterogenicity free medical journals Heterogenicity famous journals Heterogenicity Google Scholar indexed journals Challenges articles Challenges Research articles Challenges review articles Challenges PubMed articles Challenges PubMed Central articles Challenges 2023 articles Challenges 2024 articles Challenges Scopus articles Challenges impact factor journals Challenges Scopus journals Challenges PubMed journals Challenges medical journals Challenges free journals Challenges best journals Challenges top journals Challenges free medical journals Challenges famous journals Challenges Google Scholar indexed journals Biologic treatments articles Biologic treatments Research articles Biologic treatments review articles Biologic treatments PubMed articles Biologic treatments PubMed Central articles Biologic treatments 2023 articles Biologic treatments 2024 articles Biologic treatments Scopus articles Biologic treatments impact factor journals Biologic treatments Scopus journals Biologic treatments PubMed journals Biologic treatments medical journals Biologic treatments free journals Biologic treatments best journals Biologic treatments top journals Biologic treatments free medical journals Biologic treatments famous journals Biologic treatments Google Scholar indexed journals muscle weakness articles muscle weakness Research articles muscle weakness review articles muscle weakness PubMed articles muscle weakness PubMed Central articles muscle weakness 2023 articles muscle weakness 2024 articles muscle weakness Scopus articles muscle weakness impact factor journals muscle weakness Scopus journals muscle weakness PubMed journals muscle weakness medical journals muscle weakness free journals muscle weakness best journals muscle weakness top journals muscle weakness free medical journals muscle weakness famous journals muscle weakness Google Scholar indexed journals muscle biopsy articles muscle biopsy Research articles muscle biopsy review articles muscle biopsy PubMed articles muscle biopsy PubMed Central articles muscle biopsy 2023 articles muscle biopsy 2024 articles muscle biopsy Scopus articles muscle biopsy impact factor journals muscle biopsy Scopus journals muscle biopsy PubMed journals muscle biopsy medical journals muscle biopsy free journals muscle biopsy best journals muscle biopsy top journals muscle biopsy free medical journals muscle biopsy famous journals muscle biopsy Google Scholar indexed journals rheumatology articles rheumatology Research articles rheumatology review articles rheumatology PubMed articles rheumatology PubMed Central articles rheumatology 2023 articles rheumatology 2024 articles rheumatology Scopus articles rheumatology impact factor journals rheumatology Scopus journals rheumatology PubMed journals rheumatology medical journals rheumatology free journals rheumatology best journals rheumatology top journals rheumatology free medical journals rheumatology famous journals rheumatology Google Scholar indexed journals Antisynthetase syndrome articles Antisynthetase syndrome Research articles Antisynthetase syndrome review articles Antisynthetase syndrome PubMed articles Antisynthetase syndrome PubMed Central articles Antisynthetase syndrome 2023 articles Antisynthetase syndrome 2024 articles Antisynthetase syndrome Scopus articles Antisynthetase syndrome impact factor journals Antisynthetase syndrome Scopus journals Antisynthetase syndrome PubMed journals Antisynthetase syndrome medical journals Antisynthetase syndrome free journals Antisynthetase syndrome best journals Antisynthetase syndrome top journals Antisynthetase syndrome free medical journals Antisynthetase syndrome famous journals Antisynthetase syndrome Google Scholar indexed journals gastrointestinal tract articles gastrointestinal tract Research articles gastrointestinal tract review articles gastrointestinal tract PubMed articles gastrointestinal tract PubMed Central articles gastrointestinal tract 2023 articles gastrointestinal tract 2024 articles gastrointestinal tract Scopus articles gastrointestinal tract impact factor journals gastrointestinal tract Scopus journals gastrointestinal tract PubMed journals gastrointestinal tract medical journals gastrointestinal tract free journals gastrointestinal tract best journals gastrointestinal tract top journals gastrointestinal tract free medical journals gastrointestinal tract famous journals gastrointestinal tract Google Scholar indexed journals electromyography articles electromyography Research articles electromyography review articles electromyography PubMed articles electromyography PubMed Central articles electromyography 2023 articles electromyography 2024 articles electromyography Scopus articles electromyography impact factor journals electromyography Scopus journals electromyography PubMed journals electromyography medical journals electromyography free journals electromyography best journals electromyography top journals electromyography free medical journals electromyography famous journals electromyography Google Scholar indexed journals
Article Details
1. Introduction
The idiopathic inflammatory myopathies (IIMs) are a heterogeneous group of disorders characterized by muscle weakness, elevated serum creatine kinase (CK) and the presence of typical inflammation in muscle biopsy [1]. The inflammatory process frequently affects other organs including the skin, joints, lungs, gastrointestinal tract and the heart [2]. The clinical presentation and the magnitude of organ involvement vary considerably among patients diagnosed with IIMs. Previously, the IIMs were diagnosed on the basis of muscle weakness, skin findings and tissue biopsy as well as electromyography (EMG). The different IIMs were classified into dermatomyositis (DM), polymyositis (PM), inclusion body myositis (IBM) and immune-mediated necrotizing myopathy (IMNM) [3]. Overtime and based on clinical observations, two distinct clinical entities within the group of IIMs were identified and added to the classification; antisynthetase syndrome (ASS) and amyopathic dermatomyositis [3]. Due to the rarity of IIMs, only a few randomized clinical trials are available to guide the treatment decisions [4]. Currently, there are several available treatments for IIMs but none are specific [4]. The management of IIM is further complicated by the extramuscular manifestations, such as interstitial lung disease and arthritis. The rarity of IIMs, lack of targeted and tailored treatments and the heterogeneity of the clinical presentation pose significant challenges to our ability to predict clinical response to treatment and to determine prognosis [4]. The objective of our case series is to share our experience and emphasize the need for better classification system.
2. Case Presentation
2.1 Case 1
A 49-year-old, morbid obese female with newly diagnosed diabetes mellitus was admitted due to progressive proximal muscle weakness, photosensitive rash involving the face, trunk and extremities and severe pruritus (Figure 1). In physical examination a proximal muscle weakness of the upper and lower extremities was noted as well as cutaneous rash and periungal erythema. Laboratory workup was significant for creatine kinase (CK) 12688 U/L (normal limits 29-168 U/L), C-reactive protein (CRP) 41 mg/L (normal limits 0.2-5 mg/L), Aspartate aminotransferase (AST) 907 U/L (normal limits 5-34 U/L), Alanine aminotransferase (ALT) 292 U/L (normal limits 0-55 U/L). Anti-nuclear (ANA), extractable nuclear antibody (ENA) and Anti-Jo1 were negative. Skin biopsy of the abdomen demonstrated a necrolytic migratory erythema without signs of vasculitis. Magnetic Resonance Imaging (MRI) of the lower extremities documented bilateral typical inflammatory myopathy (Figure 1).
Muscle biopsy demonstrated inflammatory myopathy consistent with DM. She was diagnosed with DM and high dose of corticosteroid therapy was initiated in combination with hydroxychloroquine, topical creams and anti-histamines. The patient showed a mild improvement in her muscle weakness and skin rash. A decline in CK levels to 215 U/L was observed. She was later discharged. One month later the patient was readmitted with progressive muscle weakness. On admission the CK levels were 197 U/L. She underwent an abdominal computed tomography (CT) scan that demonstrated ovarian lesions involving both ovaries with peritoneal spread. Omental biopsy was performed and the results were consistent with ovarian carcinoma. The patient underwent total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO) and omentectomy. Adjuvant chemotherapy was given after surgery. Several months after the initiation of chemotherapy, the patient's muscle weakness resolved completely and the patient regained her full muscle strength.
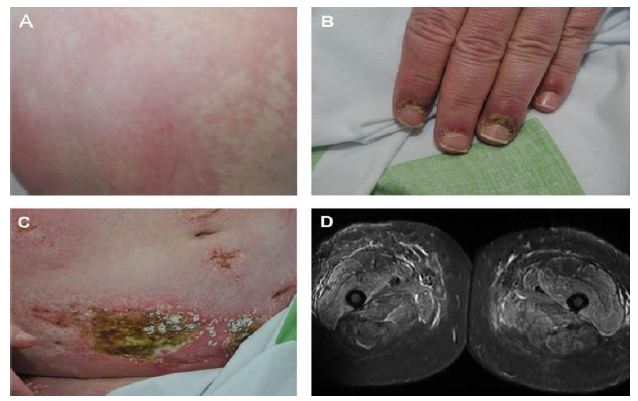
Figure 1: (A) Rash involving the back (B) periungal erythema (C) cutaneous rash with areas of necrosis on the abdominal wall (D) Magnetic resonance imaging (MRI) in Short-TI Inversion Recovery (STIR) protocol demonstrating proximal muscle involvement.
2.2 Case 2
Eighty-year-old female was diagnosed with PM prior to her visit at our clinic. The diagnosis of her PM was made based on progressive proximal muscle weakness affecting the lower extremities, elevated CK levels and typical inflammatory myopathy consistent with PM demonstrated on MRI. Electromyography (EMG) and muscle biopsy didn't reveal an inflammation, necrosis or atrophy of the muscle. Malignancy work-up including colonoscopy and total body CT scan was unrevealing. She was initially treated with high dose systemic corticosteroids in combination with different disease modifying anti-rheumatic drugs (DMARDs) including azathioprine, methotrexate (MTX) and cyclosporine. The response to these treatments was partial and resulted in mild reduction in her muscle weakness and CK levels. All DMARDs were discontinued overtime due to side effects. We initiated treatment with monthly low dose (30 grams) of intravenous immunoglobulins (IVIG) combined with tacrolimus. This regimen led to complete remission; she regained her muscle strength with normalization of her CK levels.
2.3 Case 3
48-year-old female presented to our clinic with weakness and pain in her shoulder and thigh muscles. Review of systems was significant for dry eyes and mouth as well as symmetric arthralgia affecting mainly the small joints of her hands. In physical examination a proximal muscle weakness involving the upper and lower extremities was noted as well as arthritis involving the small joints of her hands. Laboratory workup was significant for elevation in CK levels (498 U/L). Anti-nuclear (ANA), anti-Cyclic Citrullinated Peptide (CCP), anti-Jo1 and anti-Sjögren’s-syndrome-related antigen A (anti-SSA) were positive. MRI revealed an inflammation in the muscles of the hips (Figure 2). The patient was diagnosed with overlap connective tissue disease of anti-synthetase syndrome, rheumatoid arthritis and Sjögren syndrome. She was initially treated with high dose systemic corticosteroids in combination with hydroxychloroquine and MTX. Due to incomplete response and the persistence of proximal muscle weakness treatment with abatacept was initiated and led to marked improvement in her muscle strength, arthritis and sicca symptoms with associated normalization of her CK levels and overtime enabled successful tapering of her systemic corticosteroids.
2.4 Case 4
A 40 year-old male, without prior medical history, presented with skin rash compatible with DM, mechanic hands, proximal muscle weakness and peripheral arthritis. The muscle weakness progressed over a period of few months and led to severe disability interfering with activities of daily living. His laboratory workup was notable for markedly elevated CK levels with positive Anti-Jo1 and ANA in high titers. Muscle biopsy of the quadriceps muscle demonstrated minimal signs of inflammation. MRI of the lower extremities documented bilateral inflammatory myopathy mainly involving the iliopsoas and gluteus muscles (Figure 3). High resolution CT of the chest demonstrated interstitial lung disease compatible with nonspecific interstitial pneumonia (NSIP). Pulmonary function tests showed restrictive disease pattern with low lung volumes and diffusion capacity. Because of the muscle weakness, skin rash, mechanic hands, interstitial lung disease and positivity to Anti-Jo1, he was diagnosed with AAS. Work-up for underlying malignancy was unrevealing. He was initially treated with pulse corticosteroid therapy (methylprednisolone one gram per day for three consecutive days) and cyclophosphamide. Intravenous immunoglobulin (IVIG) was added to the treatment. After three months of treatment the patient didn't improve with progressive muscle weakness and persistent elevation in his CK levels. Cyclophosphamide was discontinued and treatment with rituximab was initiated. Three months later, a mild improvement in muscle strength was noted. The effects of rituximab treatment weaned off over a period of two years even when tacrolimus was added as a maintenance therapy, and eventually was discontinued. We then decided to initiate treatment with intravenous tocilizumab. No improvement was demonstrated after 3 months of monthly tocilizumab infusions. Currently, an autologous bone marrow transplantation is being considered with the patient.
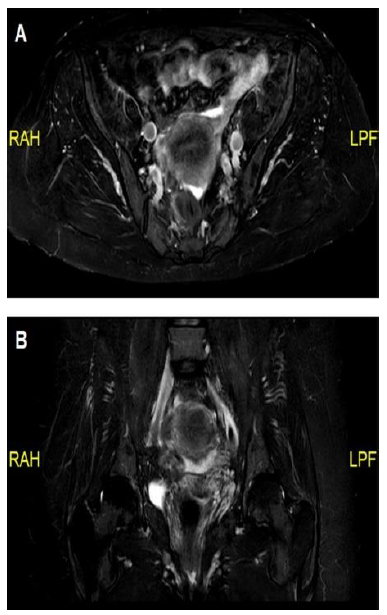
Figure 2: (A) Magnetic resonance imaging (MRI) in Dixon protocol demonstrating mild iliopsoas muscle inflammation; (B) MRI in Short-TI Inversion Recovery (STIR) protocol showing mild iliopsoas muscle inflammation.
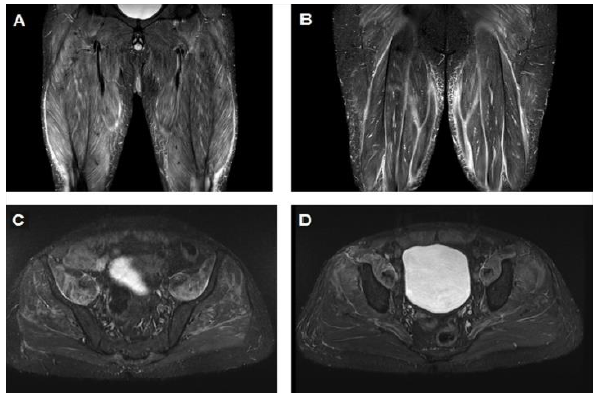
Figure 3: (A) Magnetic resonance imaging (MRI) demonstrating marked inflammation involving the quadriceps muscles; (B) MRI demonstrating marked inflammation involving the hamstrings muscles; (C) Marked inflammation of the Ileopsoas, gluteal and proximal muscles of the lower limbs; (D) Proximal muscle inflammation of the lower limbs.
2.5 Case 5
A 50-year-old male presented with dyspnea, cough and skin rash involving the hands without muscle weakness. Physical examination was notable for mechanical hands and gottron's papules. Lung exam was significant for decreased breath sounds at lung bases bilaterally with associated dry crackles. Preserved muscle strength was noted. CK levels were normal repeatdly; MRI of the arms and the thighs was negative for myositis. Serology was only positive for Anti-Jo1. CT scan of the lungs demonstrated interstitial lung disease and pulmonary function tests showed a moderate decline in diffusion capacity (DLCO) and forced vital capacity (FVC). The patient was diagnosed with Anti-synthetase syndrome. He was initially treated with high dose corticosteroids resulting in an improvement in the dyspnea, pulmonary function tests and the findings in imaging. Mycophenolate mofetil was added and corticosteroids were tapered off gradually.
3. Discussion
In this case series, we presented five cases of patients diagnosed with IIMs who had heterogeneous clinical presentation and variable response to treatment even within the same classified type of IIM (table 1). We believe that some of this heterogeneity can be attributed to the current disease classification scheme. The classification criteria of IIMs has evolved greatly since Bohan and Peter proposed their original classification system in 1975 (Table 2) [5]. Their criteria had several limitations, including the exclusion of the IBM subgroup [3, 5]. Interestingly, interstitial lung disease (ILD) was not listed as one of the cardiopulmonary manifestations in the original criteria [3].
Later, Dalakas et al. proposed a new classification system, emphasizing the importance of muscle biopsy, which since became the gold standard for the diagnosis of IIMs. The critics of these criteria were concerned with the potential pitfalls of defining IIMs subsets based on biopsy alone [6]. Over the years new myositis specific (MSAs) and myositis associated autoantibodies (MAAs) were identified. The presence of specific antibodies was found to be associated with specific clinical phenotypes with implications on prognosis. Those antibodies are though to be helpful in identifying subsets of patients with similar disease features and response to treatment [3]. The discovery of these autoantibodies led to the proposal of a serologic diagnostic approach complementary to the Bohan and Peter IIM classification to better classify the IIMs based on clinical features, serology and muscle biopsy findings [7]. Currently, this classification criteria is limited by the low availability and high cost of the methods required for identification of these autoantibodies [5]. Even when the serologic work-up is available, a large number of patients are still considered "sero-negative" and remain undefined with respect to diagnosis, prognosis, and survival [5].
Because of these limitations, the EULAR (European league against rheumatism) and ACR (American college of rheumatology) developed a new classification system for IIMs based on a probability-score model (table 3). These criteria use a clinical (muscle weakness, extra-muscular manifestations and skin rash), laboratory (Anti-Jo1 and muscle enzymes) and muscle biopsy findings to generate an aggregated score. These criteria better define the IIMs and their subgroups and specifically improve the detection rates of amyopathic DM [3]. This classification system doesn't include the other MSA and MAA antibodies in the ranking model. We believe that MSA and MAA antibodies have a prognostic utility and should be incorporated in the criteria in order to increase the specificity and further define subgroups of IIMs with similar features. Moreover, the group of MSA and MAA-negative patients is still in need of better definition and classification.
Over the last few decades, our understanding of the IIM pathogenesis has greatly evolved [8-12]. New and novel biologic drugs have been applied in various rheumatic diseases and changed the clinical outcomes of many patients. Thus, many of these drugs have been used for treatment of IIM patients. However, defining the optimal treatment regimens for the IIMs has been difficult because of the rarity of these disorders, their highly complex clinical phenotypes and heterogeneity, and the limited number of randomized, double-blind clinical trials [13-15]. The current treatment recommendations are presented in figure 4. However, those recommendations are lacking as the treatment response remain variable among IIM patients. The 5 cases we presented demonstrate the heterogenous presentation and the variable response to treatment of the IIMs. As our understanding of the pathogenesis grows, we will probably be able to sub-classify patients based on the underlying specific and dominant pathogenic pathway and tailor the treatment accordingly. More research is needed to better classify the patients and tailoring the treatment.
|
Cases |
Case 1 |
Case 2 |
Case 3 |
Case 4 |
Case 5 |
|
Age |
49 |
80 |
48 |
40 |
50 |
|
Gender |
Female |
Female |
Female |
Male |
Male |
|
Proximal muscle weakness |
Yes |
Yes |
Yes |
Yes |
No |
|
Other features at presentation |
Photosensitive rash, severe pruritus |
None |
Sicca symptoms, symmetric arthralgia affecting the small joints |
Skin rash, mechanic hands, and peripheral arthritis. |
Dyspnea, cough and gottron's papules. |
|
Positive serology |
None |
None |
ANA Anti-CCP Anti-Jo1 Anti SSA |
ANA Anti-Jo1 |
Anti-Jo1 |
|
Malignancy |
Ovarian cancer with peritoneal spread. |
None |
None |
None |
None |
|
Pulmonary involvement |
None |
None |
None |
Interstitial lung disease |
Interstitial lung disease |
|
Diagnosis |
DM |
PM |
overlap syndrome of AAS, RA and Sjögren syndrome |
AAS |
AAS |
|
Response was achieved using: |
Corticosteroids and TAH-BSO |
IVIG with tacrolimus |
Abatacept |
None |
Mycophenolate mofetil |
ANA-Anti-nuclear antibodies; Anti-CCP-Anti-cyclic citrullinated peptide; Anti-SSA-Anti-Sjögren’s syndrome related antigen A; DM-Dermatomyositis; PM-Polymyositis; RA-Rheumatoid arthritis; TAH-BSO-total abdominal hysterectomy and bilateral salpingo-oophorectomy; IVIG-Intravenous Immunoglobulin
Table 1: Patient presentation, serology, diagnosis and response to treatment.
|
1. Symmetric proximal muscle weakness determined by physical examination |
|
2. Elevation of serum skeletal muscle enzymes, including creatine kinase, aldolase, serum glutamate oxaloacetate and pyruvate transaminases, lactate dehydrogenase |
|
3. The electromyographic triad of short, small, polyphasic motor unit potentials; fibrillations, positive sharp waves, and insertional irritability; and bizarre, high-frequency repetitive discharges |
|
4. Muscle biopsy abnormalities of degeneration, regeneration, necrosis, phagocytosis, and an interstitial mononuclear infiltrate |
|
5. Typical skin rash of dermatomyositis, including a heliotrope rash and Gottron’s sign/papules |
The diagnosis of dermatomyositis is considered definite, probable and possible when skin rash is associated with 3, 2 or 1 muscular criteria, respectively.
Table 2: Bohan and Peter’s diagnostic criteria.
|
Variable |
Score |
|
|
Without muscle biopsy |
With muscle biopsy |
|
|
Age of onset of first symptom assumed to be related to the disease ≥ 18years and < 40 years |
1.3 |
1.5 |
|
Age of onset of first symptom assumed to be related to the disease ≥ 40 years |
2.1 |
2.2 |
|
Muscle weakness |
||
|
Objective symmetrical weakness, usually progressive, of the proximal upper extremities |
0.7 |
0.7 |
|
Objective symmetrical weakness, usually progressive, of the proximal lower extremities |
0.8 |
0.5 |
|
Neck flexors are relatively weaker than neck extensors |
1.9 |
1.6 |
|
In the legs, proximal muscles are relatively weaker than distal muscles |
0.9 |
1.2 |
|
Skin manifestations |
||
|
Heliotrope rash |
3.1 |
3.2 |
|
Gottron papules |
2.1 |
2.7 |
|
Gottron sign |
3.3 |
3.7 |
|
Other clinical manifestations |
||
|
Dysphagia or esophageal dysmotility |
0.7 |
0.6 |
|
Laboratory measurements |
||
|
Anti-histidyl-transfer RNA synthetase (Jo1) autoantibody present |
3.9 |
3.8 |
|
Elevated serum levels of one of the following enzymesa: creatine kinase, lactate dehydrogenase, aspartate aminotransferase or alanine aminotransferase |
1.3 |
1.4 |
|
Muscle biopsy features — presence of |
||
|
Endomysial infiltration of mononuclear cells surrounding, but not invading, myofibres |
- |
1.7 |
|
Perimysial and/or perivascular infiltration of mononuclear cells |
- |
1.2 |
|
Perifascicular atrophy |
- |
1.9 |
|
Rimmed vacuoles |
- |
3.1 |
|
Muscle biopsy available Probable idiopathic inflammatory myopathies (IIMs): aggregated score (probability ≥55% and <90%) ≥6.7 and <8.7 Definite IIMs: aggregated score (probability ≥90%) ≥8.7 Muscle biopsy not available Probable IIMs: aggregated score (probability ≥55% and <90%) ≥5.5 and <7.5 Definite IIMs: aggregated score (≥90% probability) ≥7 |
||
EULAR-European league against rheumatism; ACR-American college of rheumatology; IIMs-Idiopathic inflammatory myopathies
Table 3: The EULAR-ACR classification criteria for adult and juvenile IIMs and their major subgroups [3].
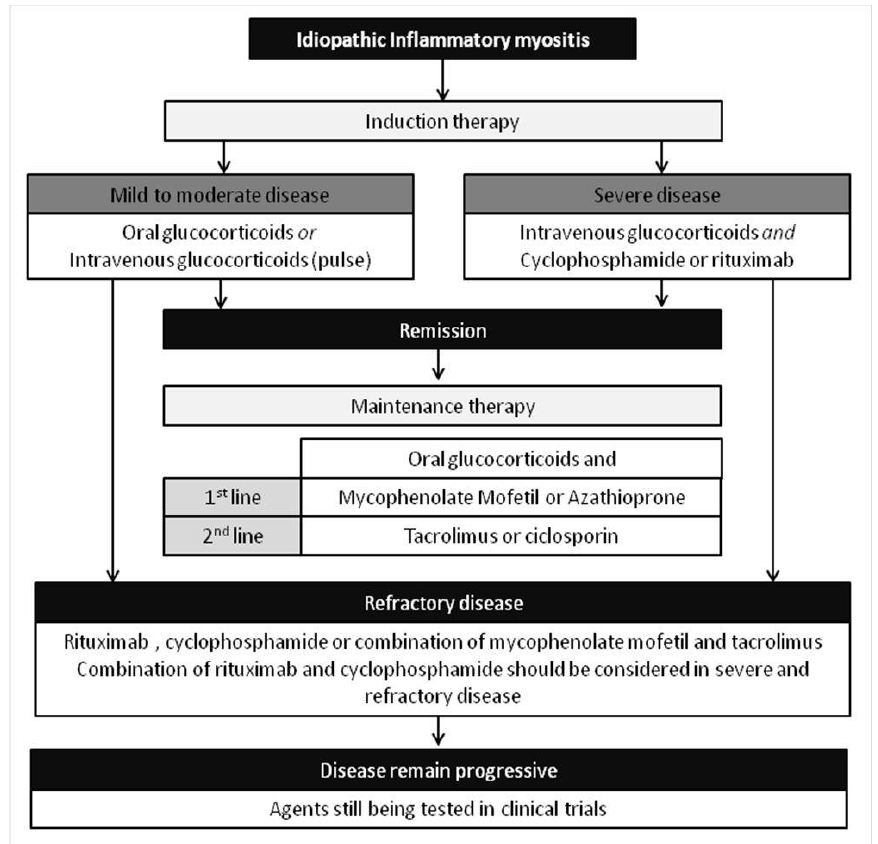
Figure 4: Current treatment algorithm for idiopathic inflammatory myopathies [3].
4. Conclusion
The current classification criteria classify the patients into homogenous groups. However, even with the current classification criteria, patients diagnosed within the same subgroup, e.g. AAS, may still have variable clinical presentations and unpredictable response to treatment, emphasizing the unmet need for better classification criteria to allow improved outcomes.
Conflict of Interests
The authors do not have any conflicts of interest to declare.
References
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 362 (2003): 971-982.
- Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC international workshop: Trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscular Disorders 14 (2004): 337-345.
- Lundberg IE, De Visser M, Werth VP. Classification of myositis. Nature Reviews Rheumatology 14 (2018): 269-278.
- Oddis CV, Aggarwal R. Treatment in myositis. Nat Rev Rheumatol 14 (2018): 279-289.
- Troyanov Y, Targoff IN, Tremblay JL, et al. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore) 84 (2005): 231-249.
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population- based cohort study. Ann Intern Med 134 (2001): 1087-1095.
- Love LA, Leff RL, Fraser DD, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 70 (1991): 360-374.
- Greenberg SA, Pinkus GS, Amato AA, et al. Myeloid dendritic cells in inclusion-body myositis and polymyositis. Muscle Nerve 35 (2007): 17-23.
- Greenberg SA, Pinkus JL, Pinkus GS, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann. Neurol 57 (2005): 664-678.
- Bilgic H, Ytterberg SR, Amin S, et al. Interleukin-6 and type I interferon- regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum 60 (2009): 3436-3446.
- Kurasawa K, Nawata Y, Takabayashi K, et al. Activation of pulmonary T cells in corticosteroid-resistant and -sensitive interstitial pneumonitis in dermatomyositis/polymyositis. Clin. Exp. Immunol 129 (2002): 541-548.
- Yamadori I, Fujita J, Kajitani H, et al. Lymphocyte subsets in lung tissues of interstitial pneumonia associated with untreated polymyositis/dermatomyositis. Rheumatol Int 21 (2001): 89-93.
- Bunch TW, Worthington JW, Combs JJ, et al. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med 92 (1980): 365-369.
- Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med 329 (1993): 1993-2000.
- Miller FW, Leitman SF, Cronin ME, et al. Controlled trial of plasma exchange and leukapheresis in polymyositis and dermatomyositis. N Engl J Med 326 (1992): 1380-1384.
