The CD4 Receptor: An Indispensable Protein in T Cell Activation and A Promising Target for Immunosuppression
Article Information
Elisa Claeys and Kurt Vermeire*
KU Leuven Department of Microbiology, Immunology and Transplantation, Rega Institute, Laboratory of Virology and Chemotherapy, B-3000 Leuven, Belgium
*Corresponding Author: Kurt Vermeire, KU Leuven Department of Microbiology, Immunology and Transplantation, Rega Institute, Laboratory of Virology and Chemotherapy, B-3000 Leuven, Belgium
Received: 23 August 2019; Accepted: 05 September 2019; Published: 09 September 2019;
Citation: Elisa Claeys and Kurt Vermeire. The CD4 Receptor: An Indispensable Protein in T Cell Activation and A Promising Target for Immunosuppression. Archives of Microbiology & Immunology 3 (2019): 133-150.
View / Download Pdf Share at FacebookAbstract
The CD4 receptor is the primary entry receptor for the human immunodeficiency virus. Besides this detrimental function, the CD4 receptor is crucial for positive selection and development of CD4+ T cells as well as for proper functioning of the immune system. During T cell activation, the CD4 receptor can fulfill an adhesion function, act as a signaling molecule and enhance the sensitivity of T cells to antigens. In addition, the CD4 receptor was suggested to be involved in differentiation towards the T helper 2 subset and in chemotaxis of T cells. In other types of immune cells, diverging functions are attributed to the CD4 receptor. The immunological importance of the CD4 receptor makes it an interesting target for immunosuppression. This is demonstrated by the immunosuppressive potential of several anti-CD4 monoclonal antibodies. These antibodies may have several modes of action, such as (1) inhibition of CD4+ T cell activation by steric hindrance of the CD4/major histocompatibility complex class II interaction resulting in antigen-specific tolerance, (2) down-modulation of the CD4 receptor, (3) switching from a pro-inflammatory T helper 1 to a more immunomodulatory T helper 2 type immune response, (4) induction of regulatory T cells and enhancement of their activity, or (5) delivery of a negative or attenuated signal into the CD4+ T cell. In addition, medicinal drugs that target CD4 are interesting alternatives for immunosuppressive treatment. The small molecule cyclotriazadisulfonamide (CADA) that down-modulates the CD4 receptor in a unique way by signal peptide-dependent inhibition of ER co-translational translocation is currently under investigation as a novel immunosuppressive drug.
Keywords
CD4 receptor; T cell activation; anti-CD4 monoclonal antibodies; cyclotriazadisulfonamide; CD4 down-modulation
CD4 receptor articles, T cell activation articles, anti-CD4 monoclonal antibodies articles, cyclotriazadisulfonamide articles, CD4 down-modulation articles
Article Details
List of abbreviations
APC: antigen-presenting cell; CADA: cyclotriazadisulfonamide; cAMP: cyclic adenosine monophosphate; CD4: cluster of differentiation 4; DAG: diacylglycerol; Foxp3: forkhead box P3; HIV: human immunodeficiency virus; IL: interleukin; IP3: 1,4,5-inositol triphosphate; ITAM: immunoreceptor tyrosine-based activation motif; LAT: linker of activated T cells; Lck: lymphocyte C-terminal Src kinase; MHC: major histocompatibility complex; NFAT: nuclear factor of activated T cells; PIP2: phosphatidylinositol biphosphate; PKC: protein kinase C; PLCγ1: phospholipase Cγ1; TCR: T cell receptor; Th: T helper; Treg: regulatory T; ZAP-70: zeta-associated protein of 70 kDa
1. Introduction
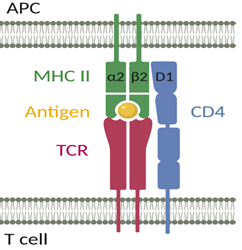
The cluster of differentiation 4 (CD4) receptor is a 55 kDa type I integral membrane protein consisting of four extracellular immunoglobulin-like domains (named D1 to D4 and defined by three disulphide-linked loop structures), a spanning transmembrane region of 22 hydrophobic amino acids and a short cytoplasmic tail of 40 amino acids [1]. Functional binding sites are distributed across the extracellular part of CD4: gp120 of human immunodeficiency virus (HIV) and the major histocompatibility complex (MHC) class II bind to the D1 domain, the T cell receptor (TCR) binds to D3, while the D4 domain is the adhesion site for interleukin (IL)-16 and is essential for CD4 dimerization [2-5].
In lymphocytic cells, the lymphocyte C-terminal Src kinase (Lck) is noncovalently linked to the CD4 receptor by interacting with two closely spaced cysteine residues in the cytoplasmic tail of CD4 [6]. Next to its signaling function in T cell activation, Lck inhibits endocytosis of the CD4 receptor by preventing the entry of CD4 into clathrin-coated pits [7]. The CD4 receptor is constitutively internalized and recycled in nonlymphoid cells, but is excluded from the endocytic pathway in lymphocytic cells [8]. Lck also targets the CD4 receptor to specialized lipid microdomains preferentially localized on microvilli, which are important in antigen recognition [9, 10].
Several cell types express the CD4 receptor: CD4+ T cells (including T helper (Th) cells, regulatory T (Treg) cells and natural killer T cells), monocytes and macrophages, natural killer cells, dendritic cells, Langerhans cells, neutrophils, basophils, eosinophils, megakaryocytes, mast cells, pro-B cells and certain cells in the central nervous system [11-19]. T cells express the highest numbers of the CD4 receptor, followed by monocytes that express already 10- to 20-fold less CD4 compared to T cells [20].
CD4-/- mice present with seriously decreased helper cell activity and marked deficiencies in MHC class II-mediated immune responses [21]. This underlines the role of the CD4 receptor in thymic selection during which double positive cells down-regulate the nonselected co-receptor after interaction with MHC, and in particular for the positive selection and development of helper T cells [22].
The CD4 receptor is also known as the primary entry receptor for HIV. Binding of a glycoprotein gp120 trimer on the surface of HIV to three CD4 receptors on the target cell induces conformational changes in gp120 that enables interaction with co-receptors [23, 24]. Co-receptor binding induces conformational changes in the transmembrane glycoprotein gp41, mediating fusion of the viral membrane with the target cell membrane [25].
CD4 receptor density plays a crucial role in the efficiency of HIV-1 infectivity, as cells with low CD4 expression require high levels of co-receptor for viral infection to occur [26]. Additionally, multimeric CD4 binding is required for HIV infection, further implying that CD4 receptor density is critical for effective HIV infection [27].
Besides its role in the positive selection and development of CD4+ T cells as well as in HIV infection, the CD4 receptor is crucial in the immune system. The most important role of CD4 is during activation of T cells, in which it can fulfill several functions. Additionally, the CD4 receptor is suggested to play a role in T helper cell differentiation and in chemotaxis of T cells towards IL-16. Also in other types of immune cells, the CD4 receptor exerts diverging functions. This paper reviews the role of the CD4 receptor in immune function and discusses interference with CD4 function by (non)depleting anti-CD4 monoclonal antibodies and by the CD4 down-modulating compound cyclotriazadisulfonamide (CADA).
2. The CD4 receptor in T cell activation
During activation of T cells, the CD4 receptor can fulfill several roles including an adhesion function, a signaling function as well as enhancement of T cell sensitivity to antigens.
2.1. Adhesion function of the CD4 receptor
The CD4 receptor was shown to exert an intercellular adhesion function during T cell activation and was therefore described as a co-receptor [28]. The CD4 receptor stabilizes the interaction between the TCR on CD4+ T cells and the MHC class II molecule on antigen-presenting cells (APCs) (Figure 1) [29, 30]. The D1 domain of CD4 can interact with both the α2 and β2 domains of MHC class II, suggesting that specifically organized CD4 and/or MHC class II oligomers play an important role in CD4+ T cell activation [31].
2.2. The CD4 receptor as signaling molecule
More important than its function in intercellular adhesion, is the signaling function of the CD4 receptor in T cell activation. Evidence for a signaling function of CD4 came from the observation that Lck positively regulates T cell activation [32]. Suboptimal TCR-induced phosphorylation and reduced calcium signaling in Th2 cells compared to Th1 cells was attributed to lower levels of the CD4 receptor [33]. Additionally, HIV-1 infection was associated with the gradual loss of responsiveness of T cells to antigens by interference with the association of Lck with the CD4 receptor and by defective recruitment of Lck into the immunological synapse [34, 35].
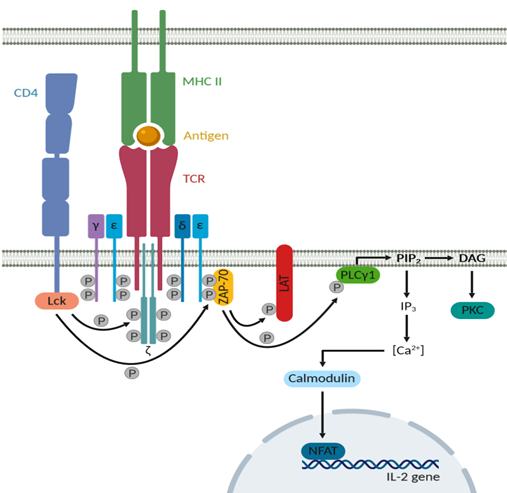
During T cell activation, the TCR is the antigen-recognition unit that interacts with the MHC class II molecule presenting the antigen. On T cells, the TCR associates with the CD3-ζ complex (Figure 2) [36]. The CD3 receptor is composed of four transmembrane polypeptide chains, a γε and a δε heterodimer. Both intracellular domains of the CD3 receptor and of the disulfide-linked ζ-dimer contain immunoreceptor tyrosine-based activation motifs (ITAMs), which are substrates for phosphorylation by protein tyrosine kinases [37]. The CD4 receptor is also associated with this TCR/CD3-ζ complex upon T cell activation, thereby bringing Lck in the proximity of the ITAMs [38]. Lck can then phosphorylate the ITAMs in the cytoplasmic tails of CD3 and of the ζ-dimer, thereby generating binding sites for proteins bearing Src homology 2 domains such as zeta-associated protein of 70 kDa (ZAP-70) [39]. Recruitment of ZAP-70 results in enhanced activation of this kinase [40]. ZAP-70 will consequently phosphorylate components of distinct downstream signaling pathways such as linker of activated T cells (LAT) and phospholipase Cγ1 (PLCγ1) [41]. LAT in turn will recruit additional signaling molecules with Src homology 2 motifs, while PLCγ1 hydrolyzes phosphatidylinositol biphosphate (PIP2), producing diacylglycerol (DAG) and 1,4,5-inositol triphosphate (IP3). DAG then activates protein kinase C (PKC), while IP3 induces calcium mobilization in the cytosol [42, 43]. Elevated intracellular calcium levels result in several effects by binding to the calcium-binding protein calmodulin. One of these effects is nuclear import of the transcription factor nuclear factor of activated T cells (NFAT) and thereby induction of transcription of the IL-2 gene [44, 45]. The final result of all these induced signaling pathways is T cell activation, characterized by increased cytosolic calcium levels, transcription factor activation, enhanced production of cytokines and massive proliferation.
Next to localizing Lck in the proximity of the TCR/CD3-ζ complex, the CD4 receptor also increases calcium mobilization from intracellular stores after engagement of CD4 by MHC class II and it counteracts the TCR-mediated increases in cyclic adenosine monophosphate (cAMP) [46]. In the absence of additional modifying signals, TCR signaling results in the accumulation of cAMP in the cytosol resulting in partial T cell activation without the induction of efficient proliferation and cytokine production [47]. CD4-mediated signals result in the reduction of cAMP levels by activating cAMP phosphodiesterases that degrade cAMP and by inhibiting adenylyl cyclase that produces cAMP from ATP. In addition, it was suggested that LAT can associate with the CD4 receptor, thereby recruiting LAT in the proximity of ZAP-70 [48].
On the other side, the CD4 receptor and Lck seem to be involved in apoptosis of T cells. CD4 crosslinking in the absence of simultaneous TCR engagement results in Lck-dependent T cell apoptosis [49]. Additionally, CD4-Lck signaling contributes in the elimination of activated T cells by Fas-mediated apoptosis [50, 51]. This is an important immunoregulatory mechanism to maintain homeostasis and prevent tissue damage.
Figure 2: The TCR and CD4 downstream signaling pathway after antigen recognition. Upon antigen recognition, the CD4 receptor associates with the TCR/CD3-ζ complex and localizes Lck in the proximity of the ITAMs on the ζ-dimer and on the γε and δε heterodimers of the CD3 receptor. These ITAMs are phosphorylated by Lck, thereby creating binding sites for ZAP-70, resulting in enhanced activity of this kinase. ZAP-70 on its turn will phosphorylate LAT and PLCγ1. LAT will recruit additional signaling molecules, while PLCγ1 hydrolyzes PIP2, which produces DAG and IP3. DAG activates PKC, while IP3 induces calcium mobilization in the cytosol. These increased calcium levels then bind to calmodulin, resulting in nuclear import of the transcription factor NFAT and thereby enhanced transcription of the IL-2 gene.
2.3. The CD4 receptor enhances T cell sensitivity
The adhesion and signaling function of the CD4 receptor stress its importance in T cell activation. It was first suggested in 1987 that CD4 can enhance T cell responsiveness and that it may be crucial in the response to suboptimal levels of antigen [52]. Later on, it was demonstrated that preventing or reducing the association of CD4 with the ligand-engaged TCR could convert typical agonists into partial agonist stimuli and that the functional role of the CD4 receptor in T cell activation varies depending upon the potency of the ligand [53, 54]. In vitro, in the presence of CD4 even one agonist peptide-MHC molecule can produce a transient increase in cytosolic calcium and as few as ten agonist peptide-MHC molecules can result in sustained calcium flux and the formation of an immunological synapse [55]. This sensitivity is highly dependent on CD4, as blocking this receptor with antibodies renders T cells unable to detect less than about 30 agonistic ligands.
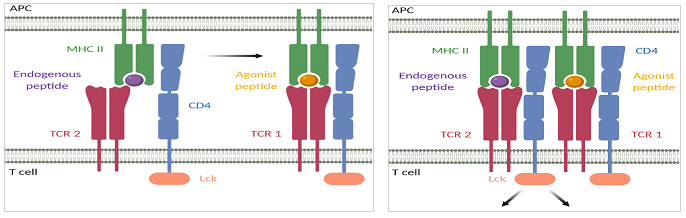
The enhancement of T cell sensitivity by the CD4 receptor can be explained by the 'pseudodimer' model (Figure 3). In this model, the binding of a first TCR (TCR1) to an agonist peptide-MHC molecule creates a hotspot for activation that can recruit another TCR (TCR2) via the CD4 receptor associated with it [56]. TCR2 then binds to an endogenous peptide-MHC molecule and the resulting stable pseudodimer complex of TCR1 binding to an agonist peptide and TCR2 binding to an endogenous peptide, triggers an activation cascade starting with Lck carried by the recruited CD4 receptor [57]. So in the presence of CD4, T cells can use endogenous peptide-MHC molecules to achieve maximal sensitivity.
Figure 3: The pseudodimer model explains enhanced T cell sensitivity in the presence of the CD4 receptor. The binding of TCR1 to an agonist peptide-MHC molecule creates a hotspot for activation that can recruit TCR2 via the CD4 receptor associated with it. TCR2 is then able to bind to an endogenous peptide-MHC molecule and the resulting stable pseudodimer complex, as shown on the right side of the figure, activates intracellular T cell signaling starting with the Lck kinase of the recruited CD4 receptor. The CD4 receptor thus enables the use of endogenous peptide-MHC molecules to achieve maximal sensitivity of T cells.
3. The CD4 receptor in T helper cell differentiation and T cell chemotaxis
Besides its role in T cell activation, the CD4 receptor is suggested to be involved in peripheral T cell differentiation towards the Th2 subset. When CD4+ T cells were activated in vitro in the presence of a nondepleting anti-CD4 monoclonal antibody, a Th2-type response was induced with elevated levels of IL-4 and IL-13 [58]. Additionally, development of the Th2 subset was impaired in CD4-/- mice [59, 60]. CD4-dependent signaling pathways would be involved in the regulation of a late checkpoint in the expansion and commitment phase of Th2 development, which is associated with resistance to activation-induced cell death [61]. Lck in turn would mediate Th2 differentiation through effects on the Th1 transcription factor T-bet and on the Th2 transcription factor GATA3 [62].
Furthermore, CD4 was identified as the primary receptor for the pro-inflammatory cytokine IL-16 that is implicated in the pathogenesis of asthma and several autoimmune diseases by recruitment of CD4+ T cells to sites of inflammation [63]. IL-16 inhibits HIV-1 and SIV infection, as well as T cell activation in the mixed lymphocyte reaction and after stimulation with anti-CD3 antibodies [64, 65]. The CD4 receptor was shown to be involved in the chemotactic response of CD4+ T cells towards IL-16 by activation of Lck, followed by increases in cytosolic calcium levels and IP3, and translocation of PKC from the cytosol to the cell membrane [66-68]. However, this function of the CD4 receptor is rather controversial as experiments in CD4-/- mice demonstrated that CD4 is not required for the functional activity of IL-16 [69]. In human and murine mast cells, the tetraspanin CD9 was identified as an alternate receptor for IL-16 [70].
4. The CD4 receptor in other types of immune cells
Although the major importance of CD4 lies in CD4+ T cells, the receptor is expressed in several other types of immune cells. In monocytes and eosinophils, CD4 would function as a chemotactic factor receptor, while in pro-B cells it transduces a signal that induces differentiation towards pre-B cells and expression of recombination-activating gene-1 and -2 [71]. In active natural killer cells, the CD4 receptor plays a role in chemotaxis towards IL-16 and in cytokine production of interferon-γ and tumor necrosis factor-α [72]. In spleen-resident dendritic cells, CD4 is involved in the priming of invariant natural killer T cells [73].
5. Interference with CD4 receptor function
The important role of the CD4 receptor in the immune system is demonstrated by the immunosuppressive potential of anti-CD4 monoclonal antibodies. The small molecule CADA down-modulates the CD4 receptor and may therefore also have immunosuppressive effects.
5.1. Anti-CD4 monoclonal antibodies
Depleting anti-CD4 monoclonal antibodies
The depleting anti-mouse CD4 monoclonal antibody GK1.5 and the depleting anti-rat CD4 monoclonal antibody OX38 were successfully used in rodent skin and small bowel allograft transplantation models [74, 75]. Several other depleting anti-CD4 monoclonal antibodies showed benefit in transplantation models with various organs. CD4+ T cells are depleted by these antibodies by antibody-dependent cellular cytotoxicity or by complement-dependent cytotoxicity [76]. Massive and long-lasting reduction of the number of CD4+ T cells is associated with a number of side effects related to general immunosuppression. Therefore, experiments with local secretion of the depleting antibodies by transgenic or transduced allografts were performed [77, 78]. Still, these depleting anti-CD4 monoclonal antibodies, while being immunosuppressive, have been found to deplete Treg cells too and thereby inhibit the induction of antigen tolerance [79].
Nondepleting anti-CD4 monoclonal antibodies
The immunosuppressive potential of depleting anti-CD4 monoclonal antibodies is obvious, but it was also shown that nondepleting anti-CD4 monoclonal antibodies could induce a permanent state of antigen-specific unresponsiveness (tolerance) in mice [80]. Nondepleting anti-CD4 monoclonal antibodies were able to control collagen-induced arthritis and experimental autoimmune encephalomyelitis in mice [81, 82]. These antibodies often did not only prevent, but also control ongoing autoimmune diseases. The effect of nondepleting anti-CD4 monoclonal antibodies was not restricted to rodent models, as it was shown in baboons that the humanized nondepleting anti-CD4 monoclonal antibody TRX1 is able to induce antigen-specific tolerance without long-term immunosuppression [83].
These nondepleting anti-CD4 monoclonal antibodies may have several mechanisms of action. Using in vitro T cell activation assays, no correlation was found between the degree of T cell activation inhibition and the specificity of the monoclonal antibodies for different regions on the CD4 receptor, even not the binding site of MHC class II [84]. CD4 down-modulation may be required for the tolerizing effect of nondepleting anti-CD4 monoclonal antibodies and it was shown in vitro that resting CD4+ T cells absolutely require Fc receptor-mediated crosslinking of a humanized nondepleting anti-CD4 antibody for CD4 to be down-modulated, while activated CD4+ T cells did not [85, 86].
In a collagen-induced arthritis model in mice, the nondepleting anti-CD4 antibody KT6 seemed to control pathogenic CD4+ T cells by switching their cytokine production from a Th1- to a Th2-like profile [81]. Additionally, production of the Th1 cytokine interferon-γ was severely reduced by the nondepleting anti-CD4 antibody RIB5/2 after allograft transplantation in rats [87]. An in vitro study with the nondepleting anti-mouse CD4 antibody YTS177.9 showed that IL-2 secretion was suppressed by this antibody through inhibition of the transcription factors NFAT and activator protein-1 (AP-1) [88].
Another mechanism that was proposed to explain the tolerizing effect of nondepleting anti-CD4 monoclonal antibodies, was by induction of differentiation of naive T cells into adaptive Treg cells or by activation of the suppressive function of Treg cells [89, 90]. The anti-mouse CD4 antibody YTS177.9 was shown to induce forkhead box P3 (Foxp3)+ Treg cells in a murine model of multiple sclerosis [82]. In contrast, YTS177.9 could inhibit proliferation of effector CD4+ T cells in an in vitro mixed lymphocyte reaction, without expansion or activation of immunosuppressive Foxp3+ Treg cells [91]. The same group demonstrated that the anti-human CD4 antibody RPA-T4 could inhibit CD4+ T cell proliferation in the mixed lymphocyte reaction in the absence of Foxp3+ Treg cells. In line with these data, YTS177.9 was able to induce tolerance in Foxp3 mutant scurfy mice and in genetically engineered mice that were depleted of Foxp3+ Treg cells [92]. On the other side, the humanized CD4-specific monoclonal antibody tregalizumab (BT-061) induces a unique phosphorylation of TCR complex-associated signaling molecules exclusively in Treg cells by recognition of a specific conformational epitope on the D2 domain of CD4 [93]. Thereby, it selectively activates the function of Treg cells without activating effector T cells. Although being very promising, tregalizumab did not show significant clinical efficacy in a phase IIb randomized, placebo-controlled trial including patients with active rheumatoid arthritis [94].
Aberrant intracellular signaling was also observed in CD4+ T cells in the presence of nondepleting anti-CD4 monoclonal antibodies. The anti-mouse CD4 antibody YTS177.9 decreases tyrosine phosphorylation of ZAP-70 and LAT, resulting in severely reduced proliferation of the responding CD4+ T cells in vitro [95]. The signaling profile of these anti-CD4-treated cells resembles that of anergic cells. Additionally, in the above-mentioned Foxp3 mutant scurfy mice, tolerance induction by YTS177.9 was associated with down-regulation of the co-stimulatory tumor necrosis factor-receptor superfamily members OX40 and CD30 [92]. Signaling through OX40 and CD30 promotes the differentiation and survival of CD4+ T cells that initiate autoimmunity in scurfy mice [96]. OX40 is also implicated in other autoimmune conditions, such as rheumatoid arthritis and multiple sclerosis [97].
Nondepleting anti-CD4 monoclonal antibodies may therefore have several modes of action, such as (1) inhibition of CD4+ T cell activation by steric hindrance of the CD4/MHC class II interaction that might result in antigen-specific tolerance, (2) down-modulation of the CD4 receptor, (3) switching from a proinflammatory Th1 to a more immunomodulatory Th2 type immune response, (4) induction of Treg cells and enhancement of their activity, or (5) delivery of a negative or attenuated signal into the CD4+ T cell [76]. These antibodies could represent a promising therapeutic approach for human autoimmune diseases and to prevent graft rejection after organ transplantation. Due to immunogenicity and the generation of anti-mouse immunoglobulins, murine monoclonal antibodies cannot be used in humans, but several humanized nondepleting anti-CD4 monoclonal antibodies have been tested in clinical trials. Many attempts were done to use these antibodies as potential immunosuppressive agents in autoimmune diseases and after organ transplantation [98]. Most of these attempts failed, mainly because there was not enough knowledge about the mechanism of action of anti-CD4-induced tolerance, about the optimal dose and about the pharmacokinetic/pharmacodynamic profile of these antibodies. One example is the monkey/human chimeric anti-CD4 monoclonal antibody Clenoliximab, that reached phase II clinical trial for the treatment of rheumatoid arthritis, but was discontinued afterwards [99].
5.2 Cyclotriazadisulfonamide (CADA)
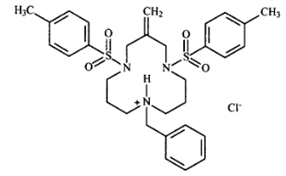
The small molecule CADA is a synthetic macrocycle that was selected from an antiviral HIV screening program of the US National Cancer Institute (Figure 4). CADA was shown to have a broad antiviral activity against different HIV strains in several T cell lines and in peripheral blood mononuclear cells [100, 101]. This antiviral effect of CADA is due to specific down-modulation of the CD4 receptor, which is the primary entry receptor for HIV. For 19 CADA analogs, it was shown that the CD4 down-modulating activity directly correlates with their anti-HIV potency [102].
This down-modulating effect of CADA was similar for surface and intracellular CD4 expression [100]. Depending on the cell type, the kinetics of CD4 down-modulation in the presence of CADA varied. The down-modulating activity of CADA is also reversible: when the compound is removed, CD4 expression is rapidly restored to normal levels. CADA does not compromise cellular viability as was demonstrated by long-term (about 1 year) CADA treatment of T cells with recovery of CD4 expression when treatment was ceased [103]. The sensitivity of the CD4 receptor to CADA is species-specific, as expression of murine CD4 was not affected by the compound, while primary T cells of macaques responded in a similar way as human T cells.
CADA does not bind directly to the extracellular part of CD4 resulting in receptor internalization, nor does it act at transcriptional level as similar mRNA levels for CD4 were obtained in the presence or absence of CADA [100]. This suggests that CADA acts at the (post)translational level. Indeed, CADA was shown to inhibit ER co-translational translocation of human CD4 by selectively binding to its signal peptide [103]. In contrast, no interaction of CADA with the murine CD4 signal peptide was observed. CADA was therefore identified as the first signal peptide-binding compound that selectively inhibits the translocation of a specific protein into the endoplasmic reticulum in a signal peptide-dependent way. The immunosuppressive potential of CADA is currently under investigation. Due to its unique mechanism of CD4 receptor down-modulation, CADA may provide a fascinating example of CD4-involved immunosuppression.
6. Conclusion
The CD4 receptor plays a crucial role in the immune system, especially during T cell activation in which it can fulfill an adhesion or signaling function and enhance sensitivity of T cells to antigens. Despite promising in vitro and in vivo immunosuppressive effects of non-depleting anti-CD4 monoclonal antibodies, translation into clinical use as immunosuppressive agents to treat autoimmune diseases and/or to prevent rejection after organ transplantation has not been successful so far. In addition, the small molecule CADA down-modulates the CD4 receptor by a unique way of signal peptide-dependent inhibition of ER co-translational translocation of the protein. The immunosuppressive potential of this compound is currently under investigation and will undoubtedly provide new insight in the field of CD4-involved immunosuppression.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- Maddon PJ, Littman DR, Godfrey M, Maddon DE, Chess L, Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell 42 (1985): 93-104.
- Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature 330 (1987): 256-259.
- Vignali DA, Vignali KM. Profound enhancement of T cell activation mediated by the interaction between the TCR and the D3 domain of CD4. Journal of immunology 162 (1999): 1431-1439.
- Liu Y, Cruikshank WW, O'Loughlin T, O'Reilly P, Center DM, Kornfeld H. Identification of a CD4 domain required for interleukin-16 binding and lymphocyte activation. The Journal of biological chemistry 274 (1999): 23387-23395.
- Wu H, Kwong PD, Hendrickson WA. Dimeric association and segmental variability in the structure of human CD4. Nature 387 (1997): 527-530.
- Shaw AS, Amrein KE, Hammond C, Stern DF, Sefton BM, Rose JK. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell 59 (1989): 627-636.
- Pelchen-Matthews A, Boulet I, Littman DR, Fagard R, Marsh M. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. The Journal of cell biology 117 (1992): 279-290.
- Pelchen-Matthews A, Armes JE, Griffiths G, Marsh M. Differential endocytosis of CD4 in lymphocytic and nonlymphocytic cells. The Journal of experimental medicine 173 (1991): 575-587.
- Foti M, Phelouzat MA, Holm A, Rasmusson BJ, Carpentier JL. p56Lck anchors CD4 to distinct microdomains on microvilli. Proceedings of the National Academy of Sciences of the United States of America 99 (2002): 2008-2013.
- Cai E, Marchuk K, Beemiller P, Beppler C, Rubashkin MG, Weaver VM, et al. Visualizing dynamic microvillar search and stabilization during ligand detection by T cells. Science 356 (2017).
- Wood GS, Warner NL, Warnke RA. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. Journal of immunology 131 (1983) :212-216.
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proceedings of the National Academy of Sciences of the United States of America 96 (1999): 5215-5220.
- Li Y, Li L, Wadley R, Reddel SW, Qi JC, Archis C, et al. Mast cells/basophils in the peripheral blood of allergic individuals who are HIV-1 susceptible due to their surface expression of CD4 and the chemokine receptors CCR3, CCR5, and CXCR4. Blood 97 (2001): 3484-3490.
- Lucey DR, Dorsky DI, Nicholson-Weller A, Weller PF. Human eosinophils express CD4 protein and bind human immunodeficiency virus 1 gp120. The Journal of experimental medicine 169 (1989): 327-332.
- Basch RS, Kouri YH, Karpatkin S. Expression of CD4 by human megakaryocytes. Proceedings of the National Academy of Sciences of the United States of America 87 (1990): 8085-8089.
- Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity 5 (1996): 527-535.
- Omri B, Crisanti P, Alliot F, Marty MC, Rutin J, Levallois C, et al. CD4 expression in neurons of the central nervous system. International immunology 6 (1994): 377-385.
- Lynch GW, Slaytor EK, Elliott FD, Saurajen A, Turville SG, Sloane AJ, et al. CD4 is expressed by epidermal Langerhans' cells predominantly as covalent dimers. Experimental dermatology 12 (2003): 700-711.
- Biswas P, Mantelli B, Sica A, Malnati M, Panzeri C, Saccani A, et al. Expression of CD4 on human peripheral blood neutrophils. Blood 101 (2003): 4452-4456.
- Collman R, Godfrey B, Cutilli J, Rhodes A, Hassan NF, Sweet R, et al. Macrophage-tropic strains of human immunodeficiency virus type 1 utilize the CD4 receptor. Journal of virology 64 (1990): 4468-4476.
- Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 353 (1991): 180-184.
- Killeen N, Davis CB, Chu K, Crooks ME, Sawada S, Scarborough JD, et al. CD4 function in thymocyte differentiation and T cell activation. Philosophical transactions of the Royal Society of London Series B, Biological sciences 342 (1993): 25-34.
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393 (1998): 648-659.
- Chan DC, Kim PS. HIV entry and its inhibition. Cell 93 (1998): 681-684.
- Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harbor perspectives in medicine 2 (2012).
- sPlatt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. Journal of virology 72 (1998): 2855-2864.
- Layne SP, Merges MJ, Dembo M, Spouge JL, Nara PL. HIV requires multiple gp120 molecules for CD4-mediated infection. Nature 346 (1990): 277-279.
- Janeway CA, Jr. The role of CD4 in T-cell activation: accessory molecule or co-receptor? Immunology today 10 (1989): 234-238.
- Miceli MC, von Hoegen P, Parnes JR. Adhesion versus coreceptor function of CD4 and CD8: role of the cytoplasmic tail in coreceptor activity. Proceedings of the National Academy of Sciences of the United States of America 88 (1991): 2623-2627.
- Gay D, Maddon P, Sekaly R, Talle MA, Godfrey M, Long E, et al. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nature 328 (1989): 626-629.
- Konig R, Shen X, Germain RN. Involvement of both major histocompatibility complex class II alpha and beta chains in CD4 function indicates a role for ordered oligomerization in T cell activation. The Journal of experimental medicine 182 (1995): 779-787.
- Abraham N, Miceli MC, Parnes JR, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature 350 (1991): 62-66.
- Itoh Y, Wang Z, Ishida H, Eichelberg K, Fujimoto N, Makino J, et al. Decreased CD4 expression by polarized T helper 2 cells contributes to suboptimal TCR-induced phosphorylation and reduced Ca2+ signaling. European journal of immunology 35 (2005): 3187-3195.
- Kanner SB, Haffar OK. HIV-1 down-regulates CD4 costimulation of TCR/CD3-directed tyrosine phosphorylation through CD4/p56lck dissociation. Journal of immunology 154 (1995): 2996-3005.
- Nyakeriga AM, Fichtenbaum CJ, Goebel J, Nicolaou SA, Conforti L, Chougnet CA. Engagement of the CD4 receptor affects the redistribution of Lck to the immunological synapse in primary T cells: implications for T-cell activation during human immunodeficiency virus type 1 infection. Journal of virology 83 (2009): 1193-1200.
- Konig R, Zhou W. Signal transduction in T helper cells: CD4 coreceptors exert complex regulatory effects on T cell activation and function. Current issues in molecular biology 6 (2004): 1-15.
- de Aos I, Metzger MH, Exley M, Dahl CE, Misra S, Zheng D, et al. Tyrosine phosphorylation of the CD3-epsilon subunit of the T cell antigen receptor mediates enhanced association with phosphatidylinositol 3-kinase in Jurkat T cells. The Journal of biological chemistry 272 (1997): 25310-8.
- Holdorf AD, Lee KH, Burack WR, Allen PM, Shaw AS. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nature immunology 3 (2002): 259-264.
- Chan AC, Irving BA, Fraser JD, Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proceedings of the National Academy of Sciences of the United States of America 88 (1991): 9166-70.
- LoGrasso PV, Hawkins J, Frank LJ, Wisniewski D, Marcy A. Mechanism of activation for Zap-70 catalytic activity. Proceedings of the National Academy of Sciences of the United States of America 93 (1996): 12165-70.
- Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. The Biochemical journal 356 (2001): 461-71.
- May WS, Lapetina EG, Cuatrecasas P. Intracellular activation of protein kinase C and regulation of the surface transferrin receptor by diacylglycerol is a spontaneously reversible process that is associated with rapid formation of phosphatidic acid. Proceedings of the National Academy of Sciences of the United States of America 83 (1986): 1281-1284.
- Corado J, Le Deist F, Griscelli C, Fischer A. Inositol 1,4,5-trisphosphate- and arachidonic acid-induced calcium mobilization in T and B lymphocytes. Cellular immunology 126 (1990): 245-54.
- Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature 383 (1996): 837-840.
- Rooney JW, Sun YL, Glimcher LH, Hoey T. Novel NFAT sites that mediate activation of the interleukin-2 promoter in response to T-cell receptor stimulation. Molecular and cellular biology 15 (1995): 6299-310.
- Zhou W, Konig R. T cell receptor-independent CD4 signalling: CD4-MHC class II interactions regulate intracellular calcium and cyclic AMP. Cellular signalling 15 (2003): 751-62.
- Feuerstein N, Firestein R, Aiyar N, He X, Murasko D, Cristofalo V. Late induction of CREB/ATF binding and a concomitant increase in cAMP levels in T and B lymphocytes stimulated via the antigen receptor. Journal of immunology 156 (1996): 4582-93.
- Bosselut R, Zhang W, Ashe JM, Kopacz JL, Samelson LE, Singer A. Association of the adaptor molecule LAT with CD4 and CD8 coreceptors identifies a new coreceptor function in T cell receptor signal transduction. The Journal of experimental medicine 190 (1999): 1517-26.
- Di Somma MM, Nuti S, Telford JL, Baldari CT. p56lck plays a key role in transducing apoptotic signals in T cells. FEBS letters 363 (1995): 101-4.
- Sharif-Askari E, Gaucher D, Halwani R, Ma J, Jao K, Abdallah A, et al. p56Lck tyrosine kinase enhances the assembly of death-inducing signaling complex during Fas-mediated apoptosis. The Journal of biological chemistry 282 (2007): 36048-56.
- Tinsley KW, Herzog D, Leitenberg D. CD4 co-receptor dependent signaling promotes competency for re-stimulation induced cell death of effector T cells. Cellular immunology 266 (2011): 200-7.
- Sleckman BP, Peterson A, Jones WK, Foran JA, Greenstein JL, Seed B, et al. Expression and function of CD4 in a murine T-cell hybridoma. Nature 328 (1987): 351-3.
- Madrenas J, Chau LA, Smith J, Bluestone JA, Germain RN. The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide-MHC molecule ligands. The Journal of experimental medicine 185 (1997): 219-29.
- Vidal K, Daniel C, Hill M, Littman DR, Allen PM. Differential requirements for CD4 in TCR-ligand interactions. Journal of immunology 163 (1999): 4811-8.
- Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature 419 (2002): 845-9.
- Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature 434 (2005): 238-43.
- Li QJ, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, et al. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nature immunology 5 (2004): 791-9.
- Stumbles P, Mason D. Activation of CD4+ T cells in the presence of a nondepleting monoclonal antibody to CD4 induces a Th2-type response in vitro. The Journal of experimental medicine 182 (1995): 5-13.
- Fowell DJ, Magram J, Turck CW, Killeen N, Locksley RM. Impaired Th2 subset development in the absence of CD4. Immunity 6 (1997): 559-69.
- Brown DR, Moskowitz NH, Killeen N, Reiner SL. A role for CD4 in peripheral T cell differentiation. The Journal of experimental medicine 186 (1997): 101-7.
- Tatari-Calderone Z, Brogdon JL, Tinsley KW, Ramezani A, Leitenberg D. CD4-dependent signaling is required for a late checkpoint during Th2 development associated with resistance to activation-induced cell death. Journal of immunology 175 (2005): 5629-36.
- Kemp KL, Levin SD, Bryce PJ, Stein PL. Lck mediates Th2 differentiation through effects on T-bet and GATA-3. Journal of immunology 184 (2010): 4178-84.
- Nicoll J, Cruikshank WW, Brazer W, Liu Y, Center DM, Kornfeld H. Identification of domains in IL-16 critical for biological activity. Journal of immunology 163 (1999): 1827-32.
- Cruikshank WW, Kornfeld H, Center DM. Interleukin-16. Journal of leukocyte biology 67 (2000): 757-66.
- Cruikshank WW, Lim K, Theodore AC, Cook J, Fine G, Weller PF, et al. IL-16 inhibition of CD3-dependent lymphocyte activation and proliferation. Journal of immunology 157 (1996): 5240-8.
- Ryan TC, Cruikshank WW, Kornfeld H, Collins TL, Center DM. The CD4-associated tyrosine kinase p56lck is required for lymphocyte chemoattractant factor-induced T lymphocyte migration. The Journal of biological chemistry 270 (1995): 17081-6.
- Cruikshank WW, Greenstein JL, Theodore AC, Center DM. Lymphocyte chemoattractant factor induces CD4-dependent intracytoplasmic signaling in lymphocytes. Journal of immunology 146 (1991): 2928-34.
- Parada NA, Cruikshank WW, Danis HL, Ryan TC, Center DM. IL-16- and other CD4 ligand-induced migration is dependent upon protein kinase C. Cellular immunology 168 (1996): 100-6.
- Mathy NL, Bannert N, Norley SG, Kurth R. Cutting edge: CD4 is not required for the functional activity of IL-16. Journal of immunology 164 (2000): 4429-32.
- Qi JC, Wang J, Mandadi S, Tanaka K, Roufogalis BD, Madigan MC, et al. Human and mouse mast cells use the tetraspanin CD9 as an alternate interleukin-16 receptor. Blood 107 (2006): 135-42.
- Cruikshank WW, Center DM, Nisar N, Wu M, Natke B, Theodore AC, et al. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proceedings of the National Academy of Sciences of the United States of America 91 (1994): 5109-13.
- Bernstein HB, Plasterer MC, Schiff SE, Kitchen CM, Kitchen S, Zack JA. CD4 expression on activated NK cells: ligation of CD4 induces cytokine expression and cell migration. Journal of immunology 177 (2006): 3669-76.
- Bialecki E, Macho Fernandez E, Ivanov S, Paget C, Fontaine J, Rodriguez F, et al. Spleen-resident CD4+ and CD4- CD8alpha- dendritic cell subsets differ in their ability to prime invariant natural killer T lymphocytes. PloS one 6 (2011): e26919.
- Rashid A, Auchincloss H, Jr., Sharon J. Comparison of GK1.5 and chimeric rat/mouse GK1.5 anti-CD4 antibodies for prolongation of skin allograft survival and suppression of alloantibody production in mice. Journal of immunology 148 (1992): 1382-8.
- Bowles MJ, Pockley AG, Wood RF. Effect of anti-CD4 monoclonal antibody administration on rat small bowel allograft survival and circulating leukocyte populations. Transplant international 13 (2000): 211-7.
- Schulze-Koops H, Lipsky PE. Anti-CD4 monoclonal antibody therapy in human autoimmune diseases. Current directions in autoimmunity 2 (2000): 24-49.
- Zhan Y, Martin RM, Sutherland RM, Brady JL, Lew AM. Local production of anti-CD4 antibody by transgenic allogeneic grafts affords partial protection. Transplantation 70 (2000): 947-54.
- Londrigan SL, Sutherland RM, Brady JL, Zhan Y, Li R, Estella E, et al. Prolonged local expression of anti-CD4 antibody by adenovirally transduced allografts can promote long-term graft survival. The journal of gene medicine 8 (2006): 42-52.
- Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood 102 (2003): 3295-301.
- Qin SX, Wise M, Cobbold SP, Leong L, Kong YC, Parnes JR, et al. Induction of tolerance in peripheral T cells with monoclonal antibodies. European journal of immunology 20 (1990): 2737-45.
- Chu CQ, Londei M. Induction of Th2 cytokines and control of collagen-induced arthritis by nondepleting anti-CD4 Abs. Journal of immunology 157 (1996): 2685-9.
- Duarte J, Carrie N, Oliveira VG, Almeida C, Agua-Doce A, Rodrigues L, et al. T cell apoptosis and induction of Foxp3+ regulatory T cells underlie the therapeutic efficacy of CD4 blockade in experimental autoimmune encephalomyelitis. Journal of immunology 189 (2012): 1680-8.
- Winsor-Hines D, Merrill C, O'Mahony M, Rao PE, Cobbold SP, Waldmann H, et al. Induction of immunological tolerance/hyporesponsiveness in baboons with a nondepleting CD4 antibody. Journal of immunology 173 (2004): 4715-23.
- Merkenschlager M, Buck D, Beverley PC, Sattentau QJ. Functional epitope analysis of the human CD4 molecule. The MHC class II-dependent activation of resting T cells is inhibited by monoclonal antibodies to CD4 regardless whether or not they recognize epitopes involved in the binding of MHC class II or HIV gp120. Journal of immunology 145 (1990): 2839-45.
- Bartholomew M, Brett S, Barber K, Rossman C, Crowe S, Tite J. Functional analysis of the effects of a fully humanized anti-CD4 antibody on resting and activated human T cells. Immunology 85 (1995): 41-8.
- Brett SJ, Rowan W, Smith M, Bartholomew M, Tite JP. Differential functional effects of a humanized anti-CD4 antibody on resting and activated human T cells. Immunology 91 (1997): 346-53.
- Sawitzki B, Kieselbach B, Fisser M, Meisel C, Vogt K, Gaestel M, et al. IFN-gamma regulation in anti-CD4 antibody-induced T cell unresponsiveness. Journal of the American Society of Nephrology 15 (2004): 695-703.
- Chirmule N, Avots A, LakshmiTamma SM, Pahwa S, Serfling E. CD4-mediated signals induce T cell dysfunction in vivo. Journal of immunology 163 (1999): 644-9.
- Oliveira V, Sawitzki B, Chapman S, Appelt C, Gebuhr I, Wieckiewicz J, et al. Anti-CD4-mediated selection of Treg in vitro - in vitro suppression does not predict in vivo capacity to prevent graft rejection. European journal of immunology 38 (2008): 1677-88.
- Becker C, Kubach J, Wijdenes J, Knop J, Jonuleit H. CD4-mediated functional activation of human CD4+CD25+ regulatory T cells. European journal of immunology 37 (2007): 1217-23.
- Mayer CT, Huntenburg J, Nandan A, Schmitt E, Czeloth N, Sparwasser T. CD4 blockade directly inhibits mouse and human CD4(+) T cell functions independent of Foxp3(+) Tregs. Journal of autoimmunity 47 (2013): 73-82.
- Mayer CT, Tian L, Hesse C, Kuhl AA, Swallow M, Kruse F, et al. Anti-CD4 treatment inhibits autoimmunity in scurfy mice through the attenuation of co-stimulatory signals. Journal of autoimmunity 50 (2014): 23-32.
- Helling B, Konig M, Dalken B, Engling A, Kromer W, Heim K, et al. A specific CD4 epitope bound by tregalizumab mediates activation of regulatory T cells by a unique signaling pathway. Immunology and cell biology 93 (2015): 396-405.
- van Vollenhoven RF, Keystone EC, Strand V, Pacheco-Tena C, Vencovsky J, Behrens F, et al. Efficacy and safety of tregalizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase IIb, randomised, placebo-controlled trial. Annals of the rheumatic diseases 77 (2018): 495-9.
- Pullar CE, Morris PJ, Wood KJ. Altered proximal T-cell receptor signalling events in mouse CD4+ T cells in the presence of anti-CD4 monoclonal antibodies: evidence for reduced phosphorylation of Zap-70 and LAT. Scandinavian journal of immunology 57 (2003): 333-41.
- Gaspal F, Withers D, Saini M, Bekiaris V, McConnell FM, White A, et al. Abrogation of CD30 and OX40 signals prevents autoimmune disease in FoxP3-deficient mice. The Journal of experimental medicine 208 (2011): 1579-84.
- Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nature reviews Immunology 4 (2004): 420-31.
- Cobbold SP. T cell tolerance induced by therapeutic antibodies. Philosophical transactions of the Royal Society of London Series B, Biological sciences 360 (2005): 1695-705.
- Mould DR, Davis CB, Minthorn EA, Kwok DC, Elliott MJ, Luggen ME, et al. A population pharmacokinetic-pharmacodynamic analysis of single doses of clenoliximab in patients with rheumatoid arthritis. Clinical pharmacology and therapeutics 66 (1999): 246-57.
- Vermeire K, Zhang Y, Princen K, Hatse S, Samala MF, Dey K, et al. CADA inhibits human immunodeficiency virus and human herpesvirus 7 replication by down-modulation of the cellular CD4 receptor. Virology 302 (2002): 342-53.
- Vermeire K, Princen K, Hatse S, De Clercq E, Dey K, Bell TW, et al. CADA, a novel CD4-targeted HIV inhibitor, is synergistic with various anti-HIV drugs in vitro. AIDS 18 (2004): 2115-25.
- Vermeire K, Bell TW, Choi HJ, Jin Q, Samala MF, Sodoma A, et al. The Anti-HIV potency of cyclotriazadisulfonamide analogs is directly correlated with their ability to down-modulate the CD4 receptor. Molecular pharmacology 63 (2003): 203-10.
- Vermeire K, Bell TW, Van Puyenbroeck V, Giraut A, Noppen S, Liekens S, et al. Signal peptide-binding drug as a selective inhibitor of co-translational protein translocation. PLoS biology 12 (2014): e1002011.