Simple ELISA Methods to Estimate Neutralizing Antibody Titers to SARS-CoV-2: IgG Quantification, the Avidity Index, and the Surrogate Virus Neutralization Test
Article Information
Victor Manuylov*, 1, Inna Dolzhikova1, Alexandra Kudryashova2, Bogdan Cherepovich2, Anna Kovyrshina1, Anna Iliukhina1, Olga Kharchenko2, Maria Semashko1, Artem Tkachuk1, Vladimir Gushchin1, Olga Borisova2
1Gamaleya National Research Center for Epidemiology and Microbiology, Moscow, Russian Federation
2Mechnikov Research Institute for Vaccines and Sera, Moscow, Russian Federation
*Corresponding author: Victor Manuylov, Gamaleya National Research Center for Epidemiology and Microbiology. Address: 18 Gamaleya st., Moscow, 1230986 Russian Federation.
Received: 26 September 2022; Accepted: 03 October 2022; Published: 11 October 2022
Citation: Victor Manuylov, Inna Dolzhikova, Alexandra Kudryashova, Bogdan Cherepovich, Anna Kovyrshina, Anna Iliukhina, Olga Kharchenko, Maria Semashko, Artem Tkachuk, Vladimir Gushchin, Olga Borisova. Simple ELISA Methods to Estimate Neutralizing Antibody Titers to SARS-CoV-2: IgG Quantification, the Avidity Index, and the Surrogate Virus Neutralization Test. Archives of Microbiology and Immunology 6 (2022): 213-220.
View / Download Pdf Share at FacebookAbstract
A total of 104 sera sampled from vaccinated (“Sputnik V”, Russia) volunteers were tested in parallel to determine different markers of humoral immunity against SARS-CoV-2. Testing was conducted using the neutralizing antibodies titer (NtAb) in the virus neutralization assay (VNA), the IgG to RBD in the quantitative ELISA (BAU/ml), the avidity index (AI) of the IgG to RBD (also in ELISA), and the titers of “neutralizing antibodies” which can block an interaction between human ACE2 and the viral RBD proteins in a competitive ELISA surrogate virus neutralization test (sVNT). The correlation coefficients between the quantitative results in the tried ELISA assays and the “true” NtAb titers in the VNA were high, with the following values: r=0.86 for BAU/ml (95% confidence interval, CI: 0.80-0.91, p<0.0001); 0.54 for the AI (95% CI: 0,38-0,67, p<0.0001); and 0.84 for the sVNT titer (95% CI:0.79-0.90). Additionally, it was found that the multiplicative index of BAU/ml × AI (which corresponds to the concentration of the high-affinity fraction of the total IgG to the RBD) gives a maximum correlation to the NtAb titers (r=0.89, 95% CI: 0.84-0.92, p<0.0001). The sensitivity and specificity, respectively, of the tested ELISA assays in recognizing the supposedly protective NtAb titer of ≥1:160 were the following: for the BAU/ml – 87.7±8% and 97.4±5%; for the (BAU/ml × AI) index – 92.3±6.5% and 97.4±5%; for the sVNT—95.4±5.1% and 89.7±9.5%. This confirms that the tested ELISA technologies have potential as a safe and cheap alternative to the classical VNA for assessing the protective force of patient immunity against SARS-CoV-2 infection in routine clinical practice.
Keywords
SARS-CoV-2, neutralizing antibodies, IgG, RBD, avidity index, surrogate virus neutralization test, protective immunity
SARS-CoV-2 articles, neutralizing antibodies articles, IgG articles, RBD articles, avidity index articles, surrogate virus neutralization test articles, protective immunity articles
Article Details
1. Introduction
The titer of virus-neutralizing antibodies (NtAb titer) is the main serological parameter that is directly associated with the protective effectiveness of humoral immunity against SARS-CoV-2 [1]. The NtAb titer is classically determined by using the virological neutralization assay (VNA), and usually refers to the final dilution of the analyzed serum (traditionally with step of two: 1:10, 1:20, 1:40, and so on), at which point the antibodies are still able to prevent live virus replication in a competent cell culture [2]. The VNA gives strong and reproducible results, but it requires special biosafety containment measures, so it cannot be implemented in a routine clinical laboratory to be available for a wide cohort of patients.
There have been many attempts to estimate the NtAb titer based on simpler and more commonly available immunochemistry methods, mainly by determining the IgG to the Spike/RBD antigens of SARS-CoV-2 and some their parameters. Here, we describe just a few of the works that have tackled this subject. Some early research attempted to establish a correlation between positive results in available commercial kits for anti-SARS-CoV-2 immunoglobulin detection, and the NtAb titers, which the authors supposed to be protective. Thus, Tang et al. [3] showed that the correlation between the SARS-CoV-2 neutralizing titer (EC50) and the results in the Roche and Abbott (both for IgM/IgG to the nucleocapsid antigen) and Euroimmun (IgG to the S1 subunit of the Spike protein) assays was 0.29, 0.47, and 0.46, respectively. The sensitivity levels of these assays for correctly recognizing the serums using an NtAb titer (EC50) of 1:32 were 100, 96, and 91%, respectively. However, the specificity for all of the assays was low: just 56, 69, and 81% of positive results in the listed tests corresponded to the samples which in fact had an NtAb titer of 1:32 or higher [3]. Similar results were obtained in [4] when comparing five commercial assays by Abbott, Euroimmun, EDI, ImmunoDiagnostics, and Roche. The authors shown that all of these tests were able to correctly identify sera with the presence of NtAb with high sensitivity but poor specificity. Indeed, 94–98% of the samples with NtAb titer ≥1:20 were recognized as “true” positive. However, depending on the kit manufacturer, only 12–56% of all positive samples had an actual titer of NtAb titer ≥1:20.
After implementing the BAU/ml international units [5], many authors tried to estimate the correlation between the NtAb titer and the quantity of the IgG to the Spike or RBD antigens. For example, in [6], it was shown that the median concentration of total antibodies to the S1-domain, at the level of 281 BAU/ml, was found in a group of patients who were immunized by non-symptomatic COVID-19 infection, and who carried a median 1:80 titer of the NtAb. In two other groups of patients who had either symptomatic COVID-19 or the full-dose vaccination, a median NtAb titer 1:160 corresponded to 769–983 BAU/ml.
One of the promising parameters for NtAb evaluation is the IgG avidity (more specifically, the affinity of particular immunoglobulins to certain RBD- and S1-epitopes [7,8]), which plays a role in the neutralization of the virus. NtAbs can bind the RBD in a way that blocks its interaction with human cell receptor ACE2 [9,10]. However, the RBD–ACE2 complex itself has an extremely high thermodynamic binding constant [11]. Therefore, the neutralizing antibody must have an affinity to RBD that is, at the very least, higher than the ACE2 protein in order to compete effectively for binding of the virus antigen. Based on this premise, a number of authors [12,13] have concluded that only IgGs with high avidity (or, more precisely, the high-affinity fraction of the pool of all IgG to the RBD, the proportion of which is designated as the avidity index (AI) [13]) are significant for the virus-neutralizing effectiveness of serum. Indeed, in a murine model, a direct association between the IgG to RBD avidity index and the NtAb titer has been shown, with a correlation coefficient of 0.75 [14]. Another piece of evidence that the IgG avidity is important for neutralizing abilities was the reported high risk of severe COVID-19 among patients who were repeatedly infected while their IgG to RBD had still a low avidity index (AI < 40%), compared to the patients with high-avidity antibodies (AI > 50%) who had, in general, been re-infected with a mild form of the virus [15].
Recently, surrogate virus neutralization tests (sVNTs) have become a popular means of determining the antibodies that can inhibit the interaction between the recombinant ACE2 and RBD in a competitive ELISA. A comparative study [16] has shown that use of commercial sVNT assays gives moderate but statistically significant correlations to the NtAb results obtained by classical VNA. Thus, the EUROIMMUN SARS-CoV-2 NeutraLISA kit demonstrates a correlation coefficient of 0.368–0.414 with the NtAb titers (depending on whether the tested cohort of the patients is convalescent or vaccinated), and the sVNT ELISA kit from GenScript had coefficients of 0.397–0.485. Furthermore, the modified home-brew sVNT assay [17] has shown a much more significant correlation (r=0.83) with the “true” NtAb titers, meaning that this technology has the potential to function as a safe ELISA alternative for the VNA.
It this study, we aimed to compare these three classes of immunochemistry methods (IgG quantification in BAU/ml, the determination of the avidity index, and the original sVNT assay) to determine whether they are able to recognize the “true” NtAb titers, and – more importantly – whether any of them can predict the presence of a protective titer in the serum of a particular patient.
2. Materials and methods
2.1 Samples
In total, 104 sera samples were studied. Sera samples were obtained from Sputnik V-vaccinated donors of anti-COVID plasma. Sera samples were obtained from donors vaccinated with the Sputnik V vaccine [18] between 1 and 12 months ago. The study was approved by the Gamaleya NRCEM Local Ethics Committee (Protocol No. 17, 3 December, 2021). No COVID-19 cases were reported by the participants before or after the vaccination, up to the sample collection date.
2.2 The virus neutralization assay (VNA) with live SARS-CoV-2 was performed as described previously [19]. Briefly, the procedure was as follows. The microneutralization test was performed in 96-well plates. Before analysis, the serum samples were inactivated at 56°C for 30 minutes. Dilutions of the serum (1:2.5, then from 1:10 to 1:2560 with a two-step process) were prepared in a DMEM culture medium with 2% heat-inactivated fetal bovine serum; the diluted sera were then mixed with 100 TCID50 of the SARS-CoV-2 virus (hCoV-19/Russia/Moscow_PMVL-1/2020), incubated for one hour at 37°C, and added to Vero E6 cells. The cells were incubated at 37ºC in 5% CO2. After 96 hours, the cytopathic effect (CPE) of the virus on the cell culture was recorded visually by assessing the disruption of the cell monolayer. If single "plaques" were detected in a well, such a well was considered to be a well with developed CPE. The highest dilution of serum that completely suppressed the CPE in at least two wells was considered as the NtAb titer. All the work with infectious SARS-CoV-2 virus was performed under biosafety level 3 (BSL-3) conditions
2.3 Determination of the IgG to RBD was performed using the commercial ELISA kit “SARS-CoV-2-ELISA-IgG plus” (MedipalTech LLC, Russia, certificate for medical use No. RZN 2021/14424, dated May 27, 2021). The design and reagent content of the kit is described in detail in [15]. Briefly, test serums as well as control samples were incubated in a final dilution of 1/100 in the microplate wells, covered by a recombinant RBD protein (an Arg319-Phe541 fragment of the SARS-CoV-2 Spike surface glycoprotein, Wuhan variant, GenBank: QHD43416.1, produced by Hytest, Russia). After the sera were incubated for 30 min (+37°C) and the plates were washed, 100 µL of monoclonal antibodies to human IgG (Sorbent LLC, Russia) conjugated with horseradish peroxidase (HRP) in a dilution of 1:40,000 were added to the wells and incubated for 30 min at +37°C. After washing, 100 μl of 33 mM citrate buffer solution (pH 4.0), containing 0.01% hydrogen peroxide and 0.5 mM 3,3',5,5'-tetramethylbenzidine, was added. After 15 min, the reaction was stopped by adding 100 μl of 0.5 M sulfuric acid. The optical density (OD) was measured in the two-wavelength mode at 450/680 680 nm. To determine the quantity of the IgG, the “First WHO International Standard for anti-SARS-CoV-2 immunoglobulin (human)”, NIBSC code: 20/136 (Dated 17/12/2020) [5], was used in the same assay. The calibration curve was based on five subsequent double dilutions. The analytical limit of the method used for IgG to RBD detection was 5 BAU/ml.
2.4 The determination of the IgG avidity index (AI) was performed using the same “SARS-CoV-2-ELISA-IgG plus” kit mentioned above. The procedure was generally the same [15], but each sample was tested in pair wells. After the first incubation of the 1/100-diluted serum with RBD on the plate, the "intact" well was filled with 150 μl of phosphate buffer saline, while 150 μl of 4M urea were added into the "denaturation" well. The plate was incubated for 10 min at +18-25°C and then washed. Next, the conjugate was added to the wells, and the following steps repeated the protocol described above. The avidity index (AI) was calculated according to the formula:
AI = (OD in the "denaturation" well / OD in the "intact" well) × 100%.
2.5 The surrogate virus neutralization test (sVNT) was based on the competitive ELISA method described in [20], but with some modifications. To sorb the recombinant ACE2 human protein (Vazyme #CG206, China) at the 96-well microplate (Corning #2592, USA), one hundred microliters of ACE2 solution (2 μg/ml in 0.1M carbonate-bicarbonate buffer, pH 9.6) were added to the wells and incubated for 19–22 hours at +4-8°C. After the incubation, the ACE2 solution was removed, and the wells were washed once using deionized water. Next, the blocking solution (0.09% sodium caseinate and 5% sucrose in 0.02M phosphate buffer with 0.05% Twin-20) was added to the wells and incubated for 2 hours at room temperature. After removing the blocking solution, the plates were dried in a clean-air laminar flow for 2 hours, then hermetically sealed in plastic bags and stored at +4-8°C before use.
For the analysis, each serum sample was diluted to 1:1, 1:10, and then with two steps from 1:20 to 1:10240. The dilution solution was composed as follows: 0.02% phosphate buffer (pH 7.2), 1% bovine serum albumin, 0.05% Twin-20, and 0.2% sodium caseinate. The negative control serum (NC) used in each test was diluted in the same way. The conjugate of the RBD with HRP (Vazyme #CG204H-00H-C1, China) was diluted 1:150, in the same solution as was used for the serum dilution. Next, the diluted samples were mixed with the RBD–HRD conjugate in equal volumes in the wells of the pre-mixing plate (without ACE2), and then incubated in the thermal shaker for 30 min at +37°C and 700 rpm.
After this first incubation, the mixture was transferred to the wells of the ACE2-sorbed plate and incubated for 20 min under the same conditions. Then, the plate was washed three times by phosphate-buffered saline (pH 7.2) with 0.05% Twin-20. Finally, 100 μl of 33 mM citrate buffer solution (pH 4.0), containing 0.01% hydrogen peroxide and 0.5 mM 3,3',5,5'-tetramethylbenzidine, was added to the wells. After 15 min at room temperature, the reaction was stopped by adding 100 μl of 0.5 M sulfuric acid. The OD was measured in the two-wavelength mode at 450/680 680 nm.
For each dilution of the same sample, the suppression coefficient (CS, meaning the suppression or inhibition of the interaction between the ACE2 and RBD in the presence of neutralizing antibodies) was calculated as:
CS = ((ODNC – ODSample) / ODNC) × 100%
where ODNC is the optical density for the negative control serum of the same dilution (see above).
The titer of antibodies that could neutralize the interaction between the ACE2 and the RBD (surrogate virus neutralization titer, sVNT) was considered as a final dilution of the serum with SC≥30%.
2.6 Statistics To calculate the correlation coefficients (r) between the quantitative characteristics being analyzed, Spearman's non-parameter criterion (two-tailed) was determined using Prism software (GraphPad, USA). To determine the consistency between groups with different occurrences of a qualitative characteristic (e.g., the proportion of the carriers with protective antibodies titer), Bayes' theorem was used. The binomial distribution was used to estimate confidence intervals (CI 95%, p-value < 0.05) for the proportion of qualitative characteristics in the groups.
3. Results
All the 104 serum samples with a known NtAb titer gained from the classical VNA with the live virus were tested, using an ELISA, for three quantitative markers of humoral immunity to SARS-CoV-2: IgG to RBD (in BAU/ml), the avidity index (AI) of the IgG to RBD, and the neutralization titer in the surrogate virus neutralization test (sVNT titer, see the Materials and Methods section for details). The results of these tests for all of the studied sera are given in Table A-2 in Supplementary File A. In this section, we determine the correlations between the results of these three ELISA methods and the “true” NtAb titers; next, we discuss how these methods could be used to assess the protective force of humoral immunity against SARS-CoV-2 in patients in the context of routine clinical practice.
3.1 IgG to RBD level
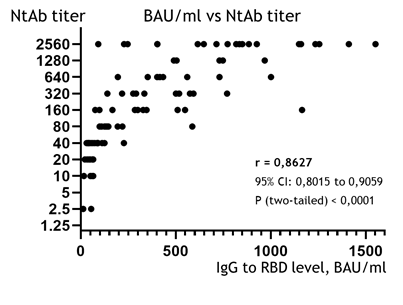
The correlation between the quantity of IgG to RBD (in BAU/ml) and the NtAb titers is presented in Figure 1. The correlation coefficient calculated using the Spearman criteria (r) was 0.8627; this shows a strong and statistically significant association between the BAU/ml and the NtAb titers in the studied group (95% CI: 0.8015 to 0.9059, p (two-tailed) < 0.0001).
Figure 1: Correlation diagram between IgG levels in BAU/ml (X-axis) and the NtAb titer, obtained from VNA (Y-axis, logarithmic) of the studied sera. Points of eight samples (from 1912 to 4137 BAU/ml, all of 1:2560 NtAb) were removed from the graph to make the main mass of points visually wider and more readable, but all the data used for the calculation of correlation are available in Table A-2 (Supplementary File A).
3.2 The avidity index and the concentration of high-affinity IgG
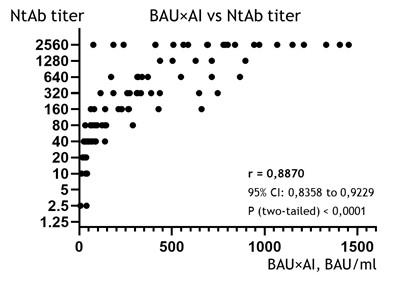
The IgG avidity index (AI) shows a weak – although still statistically reliable – correlation to the NtAb titer (r = 0.5380, 95% CI: 0,3804 to 0,6652, p < 0.0001; the diagram is not shown). This was an expected result, because the AI is not a measure of the quantity or concentration of antibodies (as are the NtAb titer and BAU/ml); rather, it represents the proportion of high-affinity IgGs (which can stay in complex with the RBD in the presence of a denaturing agent) to the total pool of the specific IgGs [21]. Nonetheless, as we know the concentration of all the IgGs to RBD expressed in BAU/ml in each sample (see the previous paragraph), it is possible to calculate the concentration of the high-affinity IgG fraction, which is assumed to offer the primary virus-neutralization effect [7,13], by simply multiplying the BAU/ml by AI. In truth, the resulting parameter, which we will henceforth designate as BAU×AI, is speculative. However, based on general reasoning, it should be proportional to the concentration of the high-affinity (and therefore, probably, neutralizing) IgGs to RBD. As such, it makes sense to look for a correlation between this artificial index of BAU×AI and the NtAb titer.
This correlation is depicted in Fig. 2, and the association between the BAU×AI and the NtAb titer (r = 0.8870, 95% CI: 0,8358 to 0,9229, p < 0,0001) was even revealed to be slightly stronger than in the case of basic BAU/ml determination (r = 0.8627, see the previous chapter). This suggests that, when additionally applied to the BAU/ml results, the avidity index can improve the evaluation of the NtAb titer.
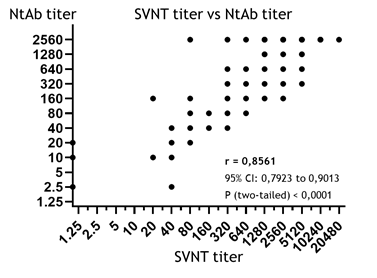
3.3 SVNT titers
As detailed in the Materials and Methods section, in our study, the sVNT titer was determined as the final dilution of the serum which was still able to markedly suppress the formation of the complex between ACE2 and RBD in vitro (coefficient of suppression, CS ≥ 30%). From this perspective, the sVNT is the method most similar to the classical VNA reaction, and is assumed to produce good results in NtAb estimation [17, 20]. However, the correlation between the sVNT and NtAb titers in the studied samples (r = 0.8561, Fig. 3) turned out to be worse compared to the correlations with BAU/ml (0.8627) and the BAU×AI index (0.8870); that said, the correlation coefficient obtained for the sVNT titers was still strong and statistically significant (95% CI: 0,7923 to 0,9013, p<0.0001). In other words, all of the three tested ELISA methods have shown acceptable results in NtAb estimation, but the most precise method was the determination of BAU×AI index. Meanwhile, the simplest method used was the routine quantification of the IgG in BAU/ml without any additional manipulations.
4. Discussion
The determination of virus-neutralizing antibodies is important for virological research or population immunity studies. However, in clinical practice, it is important not only to quantify the NtAb titer, but also to understand whether the patient carries a protective level of neutralizing antibodies. Strictly speaking, the particular NtAb titer which would guarantee protection against SARS-CoV-2 infection is unknown. It depends on the dose of the virus, the strain/variant of SARS-CoV-2, the age and physiological status of the patient, etc. As such, we can only refer to the probability of a particular NtAb titer providing protection against infection (or severe disease), in the same way that we refer to the protective effectiveness of vaccines.
As for the Omicron variant, according to our present understanding [22, 23], there seems to be no protective NtAb titer at all, in the sense of a physiologically possible concentration of IgGs that would be capable of protecting a patient from an infection with any reliable probability. Rather, for Omicron, it is more accurate to speak of an antibody titer that would be capable of protecting against a more severe case of the disease [24, 25]. However, the concept of protective humoral immunity made sense for the initial (but potentially still active) Wuhan and Delta variants, and the magnitude of the protective NtAb titer could be estimated for them with good accuracy.
Indeed, the clinical trials of the “Sputnik V” vaccine (Russia) showed that an NtAb titer of 1:44.5 (geometric mean in a group of 72 vaccinated volunteers, 95% confidence interval, CI 1:31.8 ÷ 1:62.2) protected the volunteers in the experimental group against infection with the Wuhan variant with a probability of 91.6% (CI 95% 85.6-95.2) [18]. Furthermore, it was shown [19] that it takes approximately 2.5-fold more neutralizing antibodies to inhibit the in vitro replication of the Delta variant of SARS-CoV-2 compared to the Wuhan virus. As such, we can assume that the protective titer against Delta would be 1:111 (that is, 2.5 times 44.5). Among the NtAb titers (serum dilutions) that were used in this study (see the Materials and Methods section and Table A-2 in the Supplementary Materials), the one closest was 1:160. This value can be assumed to be protective; moreover, we can presume with some certainty that an NtAb titer of 1:160 has at least a 91.6% probability of protecting the patient from infection with the Delta variant.
Next, we need to understand whether the three ELISA methods described above are capable of determining the presence of this protective level of NtAb in a sample with acceptable diagnostic sensitivity and specificity. This requires us first to set the threshold in the analyte level for each of the methods ("positive experimental result" for BAU/ml, BAU×AI index and sVNT titer), which would correspond to a "true positive" result for the protective NtAb titer (1:160 or higher) in a sample. Testing different values from Table A-2 (Supplementary Materials A) as "positive thresholds" for each of the tests and applying the Bayes theorem to them, we obtained the thresholds that have maximum "diagnostic sensitivity and specificity" in determining the "true protective titer" (Table 1):
- The concentration of the IgG to RBD ≥ 230 BAU/ml corresponds to the NtAb titer ≥ 1:160 with 87.7±8% sensitivity and 97.4±5% specificity (hereafter, CI 95% was calculated using the binomial distribution, p<0.05);
- The BAU×AI index ≥ 150 has sensitivity of 92.3±6.5% and specificity of 4±5%;
- The sVNT titer ≥ 1:320 has sensitivity of 4±5.1% and specificity of 89.7±9.5%.
In other words, if a patient has IgGs in amounts greater than 230 BAU/ml, there is a 97.4±5% probability that he or she also has a supposedly protective NtAb titer of 1:160 or higher; if a patient has a BAU×AI index ≥ 150 (a measure of the concentration of high-affinity IgGs), he or she will also have a protective NtAb titer of 1:160 or more, with a probability of 97.4±5%; finally, if there is an sVNT titer ≥ 1:320 in the sample, its donor has at least a 89.7±9.5% probability of carrying a 1:160 NtAb titer.
On the other hand, if a patient actually has a protective NtAb titer of 1:160 or more, there is an 87.7±8% probability that he or she will have IgG concentrations ≥ 230 BAU/ml; a 92.3±6.5% probability that the BAU×AI index will be 150 or more; and a 95.4±5.1% probability that the sVNT titer will be 1:320 or higher.
Table 1: Results for the determination of the “protective” NtAb titer ≥1:160 (see the main text for discussion) obtained in the studied ELISA assays and presented in a four-field-table view. Gray cells – “true” results; blank cells – “false” results; TP – true positive; FP – false positive; FN – false negative; TN – true negative. The “sensitivity” and “specificity” of the experimental tests were calculated according to the Bayes theorem as follows:
Sensitivity = TP / (TP+FN) × 100%;
Specificity = TN / (TN+FP) × 100%.
The 95% CIs for the sensitivity and specificity values were calculated using the binomial distribution.
|
Results in the VNA |
IgG to RBD (BAU/ml) |
BAU×AI index |
sVNT titer |
|||
|
“positive” |
“negative” (<230), n |
“positive” |
“negative” |
“positive” |
“negative” (<1:320), n |
|
|
(≥230), n |
(≥150), n |
(<150), n |
(≥1:320), n |
|||
|
There was a protective NtAb titer (≥1:160) |
57TP |
8FN |
60TP |
5FN |
62TP |
3FN |
|
There was not a protective NtAb titer (<1:160) |
1FP |
38TN |
1FP |
38TN |
4FP |
35TN |
|
Sensitivity of the experimental test |
87.7±8% |
92.3±6.5% |
95.4±5.1% |
|||
|
Specificity of the experimental test |
97.4±5% |
97.4±5% |
89.7±9.5% |
|||
Thus, among the methods tested, determination of the sVNT titer gives the highest sensitivity (only 4.6% of samples that have a "true" NtAb titer ≥1:160 will be recognized as false negatives), and determination of BAU/ml and the BAU×AI index recognizes the same titer with maximum specificity (only 2.6% of samples with the NtAb titer lower than a "true protective" will be misdiagnosed as positive). We believe that tests to determine the protective level of immunity should be as specific as possible, even at the expense of sensitivity, because the patient who receives a positive result should be confident in his/her protection. Therefore, we can state that the simple IgG quantification (in BAU/ml) is quite effective in assessing the levels of protective antibodies; as such, we recommend using the BAU×AI index when possible, since it has a higher sensitivity with an equally high specificity. This is the main practical result of our study.
Of course, our study had a number of limitations. We investigated only volunteers who had been vaccinated against the Wuhan variant; there were no patients in the studied group who were additionally immunized by an infection with the Delta or Omicron variants. Next, most of the participants had IgGs of rather high avidity: on average, avidity exceeded 50% in the group (see Table A-2, Supplementary A). We hypothesize (although we do not provide any evidence to support this hypothesis in this article) that testing for the BAU×AI index might be even more effective in determining the protective force of immunity in an early period of immunization, when IgGs are being produced in large amounts, but their avidity is still low (<40-50%) [15, 21].
As already noted, the obtained results cannot be directly applied to the protection force of immunity against the Omicron infection, but are still applicable regarding the Delta-like variants, which may re-emerge in the human population. In any case, the described methodology may be useful in the evaluation of arbitrary NtAb titers in routine clinical laboratories, e.g., for making decisions about preventive re-vaccination, in cases when the determined ELISA marker (BAU/ml, sVNT, etc.) has become too low to protect the patient against COVID-19 re-infection.
Declaration of Competing Interest
The authors declare no conflict of interest.
Authorship Contributions Statement
Victor Manuylov: project administration, conceptualization, writing; Inna Dolzhikova: samples collection, virus neutralization assay; Alexandra Kudryashova: ELISA, surrogate virus neutralization assay; Bogdan Cherepovich: IgG quantification, avidity index assay; Anna Kovyrshina: samples collection, virus neutralization assay; Anna Iliukhina: samples collection, virus neutralization assay; Olga Kharchenko: investigation, methodology; Maria Semashko: data processing, ethics; Artem Tkachuk: laboratory resources, editing; Vladimir Gushchin: investigation, writing; Olga Borisova: supervision, methodology, editing. All authors agree with the text of the article.
References
- Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 370 (2020):1227-1230.
- Amanat F, White KM, Miorin L, et al. An in vitro microneutralization assay for SARS-CoV-2 Serology and Drug Screening. Curr Protoc Microbiol. 58 (2020):e108.
- Tang MS, Case JB, Franks CE, et al. Association between SARS-CoV-2 Neutralizing Antibodies and Commercial Serological Assays. Clin Chem 66 (2020): 1538-1547.
- Patel EU, Bloch EM, Clarke W, et al. Comparative Performance of Five Commercially Available Serologic Assays To Detect Antibodies to SARS-CoV-2 and Identify Individuals with High Neutralizing Titers. J Clin Microbiol 59 (2021): e02257-20.
- Mattiuzzo G, Bentley EM, Hassal M, et al. Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody: Expert Committee on Biological Standartization. Geneva, 9-10 December (2020).
- Cristiano A, Nuccetelli M, Pieri M, et al. Serological anti-SARS-CoV-2 neutralizing antibodies association to live virus neutralizing test titers in COVID-19 paucisymptomatic/symptomatic patients and vaccinated subjects. Int Immunopharmacol 101 (2021): 108215.
- Piccoli L, Park YJ, Tortorici MA, et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 183 (2020): 1024-1042.e21.
- Moriyama S, Adachi Y, Sato, T, et al. Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity 54 (2021): 1841-1852.e4.
- Hoffmann M, Kleine-Weber H, Schroeder S., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181 (2020): 271-280.e8.
- Walls AC, Park YJ, Tortorici MA, et al. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 181 (2020): 281-292.e6.
- Khatri I, Staal FJT, van Dongen JJM. Blocking of the High-Affinity Interaction-Synapse Between SARS-CoV-2 Spike and Human ACE2 Proteins Likely Requires Multiple High-Affinity Antibodies: An Immune Perspective. Front Immunol 11 (2020): 570018.
- Benner SE, Patel EU, Laeyendecker O., et al. SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors. J Infect Dis 222 (2020): 1974-1984.
- Bauer G. The potential significance of high avidity immunoglobulin G (IgG) for protective immunity towards SARS-CoV-2. Int J Infect Dis 106 (2021): 61-64.
- Gaspar EB, De Gaspari E. Avidity assay to test functionality of anti-SARS-Cov-2 antibodies. Vaccine. 39 (2021):1473-1475.
- Manuylov V, Burgasova O, Borisova O et al. Avidity of IgG to SARS-CoV-2 RBD as a Prognostic Factor for the Severity of COVID-19 Reinfection. Viruses 14 (2022): 617.
- Adams O, Andrée M, Hermsen D, et al. Comparison of commercial SARS-CoV-2 surrogate neutralization assays with a full virus endpoint dilution neutralization test in two different cohorts. J Virol Methods. 307 (2022):114569.
- Kolesov DE, Sinegubova MV, Dayanova LK, et al. Fast and Accurate Surrogate Virus Neutralization Test Based on Antibody-Mediated Blocking of the Interaction of ACE2 and SARS-CoV-2 Spike Protein RBD. Diagnostics (Basel) 12 (2022): 393.
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397 (2021):671-681.
- Gushchin VA, Dolzhikova IV, Shchetinin AM, et al. Neutralizing Activity of Sera from Sputnik V-Vaccinated People against Variants of Concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow Endemic SARS-CoV-2 Variants. Vaccines (Basel). 9 (2021): 779.
- Taylor SC, Hurst B, Martiszus I, et al. Semi-quantitative, high throughput analysis of SARS-CoV-2 neutralizing antibodies: Measuring the level and duration of immune response antibodies post infection/vaccination. Vaccine 39 (2021): 5688-5698.
- Bauer G. The variability of the serological response to SARS-corona virus-2: potential resolution of ambiguity through determination of avidity (functional affinity). J Med. Virol 93 (2020): 311-322.
- Ai J, Zhang H, Zhang Y, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 11 (2022):337-343.
- Oh SJ, O SW, Choi YJ, et al. Neutralizing antibody responses in vaccinated and unvaccinated individuals infected with Omicron BA.1 variant. J Clin Virol 155 (2022): 105253.
- Bruel T, Pinaud L, Tondeur L, et al. Neutralising antibody responses to SARS-CoV-2 omicron among elderly nursing home residents following a booster dose of BNT162b2 vaccine: A community-based, prospective, longitudinal cohort study. EClinicalMedicine 51 (2022):101576.
- Gallichotte EN, Nehring M, Stromberg S, et al. Impact of Prior Infection on SARS-CoV-2 Antibody Responses in Vaccinated Long-Term Care Facility Staff. mSphere. 7 (2022): e0016922.