Reversible Posterior Leukoencephalopathy Syndrome in Patients Undergoing Chemotherapy for Solid Tumors. A Case Report and Review of the Literature
Article Information
Konstantinos Tsapakidis1*, Vasileios Papadopoulos1, Tsoukalas Nikolaos2, Prodromos Michailidis1, Alexandra Markou1, Konstantinos Kamposioras3
1Department of Medical Oncology, University Hospital of Larissa, Larissa, Greece
2Department of Oncology, 401 Military Hospital of Athens, Athens.
3Department of Medical Oncology, The Mid Yorkshire Hospitals NHS Trust, Wakefield, UK
*Corresponding Author: Konstantinos Tsapakidis MD, PhD, Department of Medical Oncology, University Hospital of Larissa, Mezourlo 41110, Larissa, Greece
Received: 14 June 2020; Accepted: 30 June 2020; Published: 10 July 2020
Citation: Konstantinos Tsapakidis, Vasilis Papadopoulos, Tsoukalas Nikolaos, Prodromos Michailidis, Chrysoula Doxani, Konstantinos Kamposioras. Reversible Posterior Leukoencephalopathy Syndrome in Patients Undergoing Chemotherapy for Solid Tumors. A Case Report and Review of the Literature. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 200-221.
View / Download Pdf Share at FacebookAbstract
Introduction: Reversible posterior leukoencephalopathy syndrome (RPLS) is a clinical entity of subtle onset of headaches, seizures, impaired vision and usually acute hypertension associated with characteristic neuroimaging findings of subcortical oedema affecting the posterior cerebral circulation. In the last two decades the causative relationship of cytotoxic and targeted antineoplastic agents with RPLS is increasingly recognized among cancer patients.
Material and Methods: Herein we present a case of advanced gastric cancer that developed RPLS after treatment with the combination of chemotherapy and Trastuzumab. A comprehensive review of the English literature and the association of cytotoxic agents used in the treatment of solid tumors, with RPLS is analyzed.
Results: 65 cases, median age 54.2 years, mainly female (83%) developed RPLS after chemotherapy-based treatment. Colorectal and lung cancer was the most frequent diagnosis, while platinum and gemcitabine based treatment was commonly related with the syndrome. Hypertension, seizures, headache and visual disturbance were the usual presenting symptoms. In the majority of the cases symptoms improved partially or completely in average in average 6.5 days after conservative management. Complete radiologic resolution of the symptoms was observed in 4.2 weeks in 57,5% of the cases and partial improvement in 2.6 weeks (42,5% of the cases).
Conclusions: Combination or single-agent chemotherapy as well as novel anticancer drugs are associated with RPLS. Clinicians need to have a high index of suspicion and the combination of the clinical picture with the characteristic neuroimaging findings can help in the prompt diagnosis. RPLS can be reversible with appropriate supportive treatment and discontinuation of the causative factors.
Keywords
Reversible posterior leucoencephalopathy (RPLS); Posterior reversible encephalopathy syndrome (PRES); Magnetic resonance imaging; hypertension; Chemotherapy.
Reversible posterior leucoencephalopathy (RPLS) articles, Posterior reversible encephalopathy syndrome (PRES) articles, Magnetic resonance imaging; hypertension articles, Chemotherapy articles
Reversible posterior leucoencephalopathy (RPLS) articles Reversible posterior leucoencephalopathy (RPLS) Research articles Reversible posterior leucoencephalopathy (RPLS) review articles Reversible posterior leucoencephalopathy (RPLS) PubMed articles Reversible posterior leucoencephalopathy (RPLS) PubMed Central articles Reversible posterior leucoencephalopathy (RPLS) 2023 articles Reversible posterior leucoencephalopathy (RPLS) 2024 articles Reversible posterior leucoencephalopathy (RPLS) Scopus articles Reversible posterior leucoencephalopathy (RPLS) impact factor journals Reversible posterior leucoencephalopathy (RPLS) Scopus journals Reversible posterior leucoencephalopathy (RPLS) PubMed journals Reversible posterior leucoencephalopathy (RPLS) medical journals Reversible posterior leucoencephalopathy (RPLS) free journals Reversible posterior leucoencephalopathy (RPLS) best journals Reversible posterior leucoencephalopathy (RPLS) top journals Reversible posterior leucoencephalopathy (RPLS) free medical journals Reversible posterior leucoencephalopathy (RPLS) famous journals Reversible posterior leucoencephalopathy (RPLS) Google Scholar indexed journals Posterior reversible encephalopathy syndrome (PRES) articles Posterior reversible encephalopathy syndrome (PRES) Research articles Posterior reversible encephalopathy syndrome (PRES) review articles Posterior reversible encephalopathy syndrome (PRES) PubMed articles Posterior reversible encephalopathy syndrome (PRES) PubMed Central articles Posterior reversible encephalopathy syndrome (PRES) 2023 articles Posterior reversible encephalopathy syndrome (PRES) 2024 articles Posterior reversible encephalopathy syndrome (PRES) Scopus articles Posterior reversible encephalopathy syndrome (PRES) impact factor journals Posterior reversible encephalopathy syndrome (PRES) Scopus journals Posterior reversible encephalopathy syndrome (PRES) PubMed journals Posterior reversible encephalopathy syndrome (PRES) medical journals Posterior reversible encephalopathy syndrome (PRES) free journals Posterior reversible encephalopathy syndrome (PRES) best journals Posterior reversible encephalopathy syndrome (PRES) top journals Posterior reversible encephalopathy syndrome (PRES) free medical journals Posterior reversible encephalopathy syndrome (PRES) famous journals Posterior reversible encephalopathy syndrome (PRES) Google Scholar indexed journals Magnetic resonance imaging articles Magnetic resonance imaging Research articles Magnetic resonance imaging review articles Magnetic resonance imaging PubMed articles Magnetic resonance imaging PubMed Central articles Magnetic resonance imaging 2023 articles Magnetic resonance imaging 2024 articles Magnetic resonance imaging Scopus articles Magnetic resonance imaging impact factor journals Magnetic resonance imaging Scopus journals Magnetic resonance imaging PubMed journals Magnetic resonance imaging medical journals Magnetic resonance imaging free journals Magnetic resonance imaging best journals Magnetic resonance imaging top journals Magnetic resonance imaging free medical journals Magnetic resonance imaging famous journals Magnetic resonance imaging Google Scholar indexed journals hypertension articles hypertension Research articles hypertension review articles hypertension PubMed articles hypertension PubMed Central articles hypertension 2023 articles hypertension 2024 articles hypertension Scopus articles hypertension impact factor journals hypertension Scopus journals hypertension PubMed journals hypertension medical journals hypertension free journals hypertension best journals hypertension top journals hypertension free medical journals hypertension famous journals hypertension Google Scholar indexed journals Chemotherapy articles Chemotherapy Research articles Chemotherapy review articles Chemotherapy PubMed articles Chemotherapy PubMed Central articles Chemotherapy 2023 articles Chemotherapy 2024 articles Chemotherapy Scopus articles Chemotherapy impact factor journals Chemotherapy Scopus journals Chemotherapy PubMed journals Chemotherapy medical journals Chemotherapy free journals Chemotherapy best journals Chemotherapy top journals Chemotherapy free medical journals Chemotherapy famous journals Chemotherapy Google Scholar indexed journals Colorectal articles Colorectal Research articles Colorectal review articles Colorectal PubMed articles Colorectal PubMed Central articles Colorectal 2023 articles Colorectal 2024 articles Colorectal Scopus articles Colorectal impact factor journals Colorectal Scopus journals Colorectal PubMed journals Colorectal medical journals Colorectal free journals Colorectal best journals Colorectal top journals Colorectal free medical journals Colorectal famous journals Colorectal Google Scholar indexed journals lung cancer articles lung cancer Research articles lung cancer review articles lung cancer PubMed articles lung cancer PubMed Central articles lung cancer 2023 articles lung cancer 2024 articles lung cancer Scopus articles lung cancer impact factor journals lung cancer Scopus journals lung cancer PubMed journals lung cancer medical journals lung cancer free journals lung cancer best journals lung cancer top journals lung cancer free medical journals lung cancer famous journals lung cancer Google Scholar indexed journals novel anticancer drugs articles novel anticancer drugs Research articles novel anticancer drugs review articles novel anticancer drugs PubMed articles novel anticancer drugs PubMed Central articles novel anticancer drugs 2023 articles novel anticancer drugs 2024 articles novel anticancer drugs Scopus articles novel anticancer drugs impact factor journals novel anticancer drugs Scopus journals novel anticancer drugs PubMed journals novel anticancer drugs medical journals novel anticancer drugs free journals novel anticancer drugs best journals novel anticancer drugs top journals novel anticancer drugs free medical journals novel anticancer drugs famous journals novel anticancer drugs Google Scholar indexed journals solid tumors articles solid tumors Research articles solid tumors review articles solid tumors PubMed articles solid tumors PubMed Central articles solid tumors 2023 articles solid tumors 2024 articles solid tumors Scopus articles solid tumors impact factor journals solid tumors Scopus journals solid tumors PubMed journals solid tumors medical journals solid tumors free journals solid tumors best journals solid tumors top journals solid tumors free medical journals solid tumors famous journals solid tumors Google Scholar indexed journals subcortical oedema articles subcortical oedema Research articles subcortical oedema review articles subcortical oedema PubMed articles subcortical oedema PubMed Central articles subcortical oedema 2023 articles subcortical oedema 2024 articles subcortical oedema Scopus articles subcortical oedema impact factor journals subcortical oedema Scopus journals subcortical oedema PubMed journals subcortical oedema medical journals subcortical oedema free journals subcortical oedema best journals subcortical oedema top journals subcortical oedema free medical journals subcortical oedema famous journals subcortical oedema Google Scholar indexed journals
Article Details
1. Introduction
Reversible Posterior Leukoencephalopathy Syndrome (RPLS) is a clinical condition characterized by headaches, seizures, impaired vision, acute hypertension and characteristic neuroimaging findings, especially posterior cerebral white matter edema [1]. It was initially described as a syndrome in 1996 and since then this clinical entity has been increasingly reported in the literature. Nevertheless, the lack of specific diagnostic criteria makes the identification of the syndrome a clinical challenge. Moreover, in the literature RPLS and Posterior reversible encephalopathy syndrome (PRES) are used interchangeably, highlighting the lack of consensus in the description of this clinical entity.
RPLS has been related with a variety of clinical conditions including malignancy and antineoplastic agents (Table 1) [1-13] . Herein we present a case of RPLS in a patient with gastric cancer treated with combination of chemotherapy and Trastuzumab. A comprehensive review of the literature in the relation of RPLS and chemotherapeutic agents in solid tumors is further performed.
|
1. Hypertensive encephalopathy [1] |
|
2. Immunosuppressive treatment - Cyclosporine A[2] - Interferon-alpha [1] |
|
3. Antineoplastic agents - Cisplatin and other platinum-based agents [3] - Gemcitabine[4] - Bevacizumab and targeted agents[5] |
|
4. Renal diseases - Acute or chronic renal diseases[6] - Thrombotic thrombocytopenic purpura[7] - Hemolytic and uremic syndrome[8] |
|
6. Autoimmune disease - Systemic lupus erythematosus[11] - Systemic sclerosis[12] - Wegener’s granulomatosis[12] |
|
7. Dexamethasone[13] |
Table 1: Medical conditions associated with reversible posterior leukoencephalopathy syndrome.
2. Case Report
We report a case of a 58 year old woman, initially treated with partial gastrectomy (Billroth II) and adjuvant chemoradiotherapy [14] for a for a poorly differentiated, intestinal type gastric adenocarcinoma stage IIIA (pT2Ν3M0). Ten months after treatment completion the patient relapsed both locally and systemically with bilateral lung metastases. Immunohistochemistry confirmed Her 2 positive (3+) staining and the patient started on first line chemotherapy with modified DCF regime (Docetaxel 40 mg/m2 on day 1, Cisplatin 40 mg/m2 on day 1 and 5FU 400 mg/m2 bolus on day 1 and followed by 1000 mg/m2 continuous infusion over 24 hours daily on days 1 and 2, repeated every 14 days) in combination with trastuzumab (6 mg/kg body weight as loading dose on day 1 and after 4 mg/kg body weight on day 1 repeated every 14 days). On day ten post cycle two, she presented at the Emergency Department of our Hospital with tonic-clonic epileptic seizures and throbbing headache. The physical examination was unremarkable while the neurological examination did not reveal any focal deficit. The main clinical and laboratory findings were normal, besides an elevated blood pressure of 177/94 mmHg and respiratory alkalosis from the arterial blood gas. MRI of the brain followed which revealed bilateral subcortical oedema at the occipital and parietal lobes (Figure 1). There were no signs of brain metastases, hemorrhage, or vascular infarction. Lumbar puncture was performed to exclude leptomeningeal disease and/or infection. The cerebrospinal fluid examination was normal (normal physical characteristics, no cancer cells present, normal biochemistry, negative microscopic examination tests for HSV1, HSV2, CMV and EBV). The clinico-radiographic diagnosis was consistent with RPLS. Patient was treated symptomatically with gradual improvement of her mental status, without any residual deficit. Brain MRI performed 4 weeks after this episode revealed that the subcortical edema had resolved (Figure 2). The patient decided against any further chemotherapy and she was offered palliative care support.
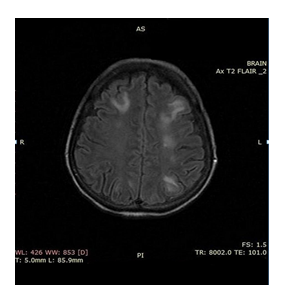
Figure 1: Magnetic resonance images of the brain showing diffuse lessions of abnormal magnetic signal mainly in subcortical white matter and in both cerebral hemispheres.
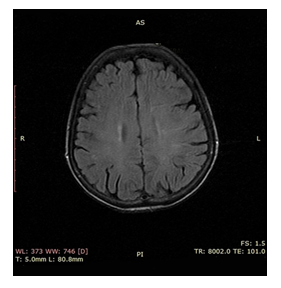
Figure 2: Follow up MRI of brain 4 weeks later showing complete response of pathological lesions.
3. Methods
We searched MEDLINE for papers using the following key words: “reversible posterior leucoencephalopathy syndrome”, “posterior reversible encephalopathy syndrome”, “cancer”, “malignancy”, and “chemotherapy”. Reference lists in the retrieved papers were checked to identify any other published data. Cases were included if there was both clinical and radiological evidence of RPLS, related to chemotherapeutic agents used to treat solid tumors. Only papers written in English language and cases with patients older than 16 years were included in the analysis. Data about the primary tumor, the chemotherapeutic agents used and the intention of the treatment, the age, gender, presenting symptoms and the number of cycles given before the development of the symptoms as well as the clinical and radiologic outcome were analyzed.
To find out if there is any correlation regarding differences between the two independent groups (males vs. females) and blood pressure in patients presented with RPLS we used Mann–Whitney test to compare them. Inferences were made at the 0.05 level of significance with no correction for multiple comparisons.
4. Results
Table 2 [3, 4, 15-67] summarizes the 67 cases, including ours, that were identified in the literature review for patients experiencing symptoms of RPLS when they were exposed to chemotherapy drugs with or without the combination of monoclonal antibodies (bevacizumab [17, 18, 22, 25, 27, 28, 37-39, 46, 51, 52, 55, 56, 58, 59, 63], trastuzumab [45, 65] or zib-aflibercept [60]) or Tyrosine Kinase Inhibitors (pazopanib [63] or erlotinib [4]). Table 3 depicts the drugs that are commonly related with the syndrome. Of the 57 cases where the treatment intention could be assessed, 46 patients (81%) were treated with palliative chemotherapy [4, 15, 17, 18, 20-25, 27-38, 41-46, 49, 51-53, 55-62, 64, 66], 9 patients had adjuvant chemotherapy [16, 19, 26, 39, 40, 47, 48, 54, 65], one neoadjuvant [67] and one treated with curative intent for testicular cancer [50]. Colorectal adenocarcinoma was the most frequent tumor type (n=19) [17, 18, 20, 22, 25, 28, 29, 37, 39, 43, 53, 54, 57-59, 61, 63, 64], followed by lung cancer (n=14; 11 NSCLC [15, 23, 24, 48, 51, 56, 60, 63, 67], 3 SCLC [31, 47, 66]), breast (n=5) [27, 44, 49, 52, 65], pancreatic (n=5) [4, 35, 40, 51, 63], urothelial bladder cancer (n=3) [21, 36, 41], sarcomas (n=3) [3, 42, 63], ovarian (n=4; 2 adenocarcinomas [54, 63] and 2 germ cell tumor (GCT) [16, 19]), gastric cancer (n=3) [34, 45], melanoma (n=2) [62, 63], glioblastoma (n=1) [38], fallopian tube (n=1) [26], cervical (1) [33], intraperitoneal mesothelioma (n=1) [30], cholangiocarcinma (n=1) [46], gall bladder (n=1) [32] and non-seminomatous GCT (n=1) [50].
In 37 cases, chemotherapeutic agents were used alone while in the rest 29 cases they were combined with targeted agents, mainly bevacizumab (Table 3). Gemcitabine was the most common agent as a single drug to cause RPLS. Platinum compounds were the most common drugs to be related with RPLS (26/37 cases), especially cisplatin (14/37). Gemcitabine was the second most common drug (12 cases) but in 7 cases it was combined with a platinum agent. In the majority of the cases (67.8%) the syndrome did appear in the first 3 cycles and in almost half of the cases (49%) in the first 2 cycles of treatment. There was a wide range though when the syndrome could present, varying between 1 and 14 cycles.
Table 2: Patients with RPLS after chemotherapy. BP: Blood Pressure; M: Male; cl: clinical response, N/A: Not assessed; MRI: Magnetic Resonance Imaging, CR: complete response, GCT: Germ Cell tumor; PVB: cisplatin, vinblastine, bleomycin; d: days; w: weeks; F: female; CRC: colorectal cancer, FOLFOX: 5-fluorouracil, leucovorin, Oxaliplatin, FOLFIRI: 5-fluorouracil, leucovorin, Irinotecan; PR: partial response; XELOX capecitabine, oxaliplatin; NSCLC: non small cell lung cancer; SCLC: small cell lung cancer; NSGCT: Non seminomatous germ cell tumor; BEP: bleomycin, etoposide, cisplatin; TCH – Docetaxel, Carboplatin, Trastuzumab.
Table 3: Chemotherapy drugs related with RPLS.
There was a significant prevalence of female patients presenting with the syndrome (Table 4). Indeed, 83% of the cases were women with median age of 53.3 years (range 17-74 years) while the median age of the 11 men was 58.8 years (range 23-81). In the majority of the cases patients presented with elevated blood pressure (BP). Systolic pressure higher than 140 mmHg and/or diastolic pressure higher than 90 mmHg was regarded as hypertension [68]. 44 of the 51 patients (86.3%) with recorded BP, presented with elevated BP. The mean systolic BP was 166.9 mmHg and the mean diastolic was 96.8 mmHg. There was no difference in BP between males and females (Table 4).
|
No. patients |
65 |
|
Males (%)† Females (%) |
11 (16.9) 54 (83.1) |
|
Mean age, years (SD) |
54.2 (±14.5) |
|
Male (n =11) ‡ Female (n = 54) |
58.8 (±14.5) 53.3 (±14.5) |
|
Mean systolic blood pressure, mmHg (SD) |
166.92 (±35.1) |
|
Males § Females |
153.8 (±34) 170.1 (±35) |
|
Mean diastolic blood pressure, mmHg (SD) |
97.1 (±19.06) |
|
Males¶ Females |
90.25 (±19.35) 98.53 (±18.94) |
Table 4: Clinical characteristics of patients presented with RPLS. †P <0.05 for gender difference with binomial test assuming 50% were women. ‡ No mean age difference between men and women (P = 0.314) (Mann–Whitney test). §No difference in systolic blood pressure between men and women (P = 0.225) (Mann–Whitney test). ¶No difference in diastolic blood pressure between men and women (P = 0.384) (Mann–Whitney test.).
The presenting symptoms and the frequency of RPLS are summarized in Table 5. Although in the majority of the cases the symptoms improved after conservative management, 2 patients unfortunately died in 2 [43] and 3 [61] days after initial admission while three more patients were reported to die in a matter of few days to weeks post presentation due to general deterioration and disease progression [4, 20, 21]. In 23 case (63.9%) of the cases symptoms resolved completely in average of 6.5 days while in 36.1 % of the cases the symptoms partially resolved in 6.4 days. The radiologic resolution of the symptoms was assessed in 40 cases. In 23 cases (57.5%) there was complete resolution in MRI in 4.2 weeks, while in the rest 17 patients (42.5%) the MRI findings partially regressed in 2.6 weeks.
|
Presenting Symptoms |
Frequency |
|
Seizures |
55.4% |
|
Headache |
37.5% |
|
Visual disturbance |
30.4% |
|
Altered mental status |
23,2% |
|
Nausea/vomiting |
16.1% |
|
Speech disturbances |
12.5% |
|
Lethargy |
8.9% |
|
Coma/ semicomatose |
3.6% |
|
Abdominal pain |
3.6% |
|
Weakness |
3.6% |
Table 5: Presenting Symptoms of RPLS.
5. Discussion
RPLS is an increasingly recognized clinical syndrome related with a variety of clinical conditions and medications (Table 1). The pathophysiologic mechanisms underlying the syndrome are poorly understood, but it appears to be related to disordered cerebral autoregulation and endothelial dysfunction leading to the characteristic cerebral oedema seen in T2-weighed MRI imaging [1, 69] . This also explains the observation that platinum based chemotherapy with or without antiangiogenic agents are frequently related with RPLS (Table 2). It is well known that platinum agents damage vascular endothelial cells, which can lead to abnormal fluctuations in blood pressure and disrupt the blood brain barrier and axonal swelling [50, 70].
To our knowledge this is the first report where RPLS is related with the combination of DCF and Trastuzumab. Nevertheless, the combination of Trastuzuamb with chemotherapy has been previously reported to cause the syndrome [45, 65]. It is difficult to assess with certainty if the monoclonal antibody itself, the chemotherapeutic agents or the combination have precipitated the event. Trastuzumab blocks tumor angiogenesis by decreasing the production of VEGF and activating antiangiogenic factors, while it can also increase the blood pressure [71]. Besides cisplatin, 5-fluorouracil has been also linked to RPLS either alone [54] or in combination with other agents [17, 18, 28, 45]. Finally, docetaxel has also been related with RPLS when it was combined with Trastuzumab and a platinum compound as in our case [65].
Our case presented with symptoms matching two of the most common clinical findings for RPLS, seizures and headache (Table 5). The brain MRI revealed the characteristic white matter oedema of the occipital and parietal lobes. Even though RPLS was high in the list of differential diagnosis, we tried to rule out other medical conditions like a stroke, demyelinating disorders, encephalitis, leptomeningeal metastases which may have similar presentation [72, 73]. Neuroimaging with MRI is crucial for the diagnosis of RPLS and lumbar puncture can be helpful to exclude any other possible causes.
The role of bevacizumab and novel targeted ant-cancer agents in the development of RPLS have been reviewed recently [5, 74]. Our scope was to review the literature considering the chemotherapy agents that seem to be related with the syndrome. To our knowledge this is the most comprehensive review including cases with solid tumors treated with chemotherapy and developed RPLS. RPLS is characterized by the combination of typical clinical symptoms (Table 5) and the characteristic neuroimaging findings. Cases where the clinical picture was quite typical for the syndrome but the CT or MRI findings were missing, were not included in the analysis [75, 76]. Another case where leucoencephalopathy was evidenced 2 months after the initial symptoms was also excluded [77]. Nevertheless, this raises the question if the lack of MRI confirmation is related to a completely different clinical entity where the findings are not expected to occur, a variant of RLPS where the brain changes may happen sometime after the clinical presentation or the need of more sensitive methods to better characterize the neuronal changes, like proton MR spectroscopy [9] and magnetic resonance angiography [78] .
In 24 cases both CT scan and MRI were used during the initial investigation. In 58% of the cases the CT scan was normal which obviously highlights that MRI should be the diagnostic imaging of choice. Nevertheless, this rate is much higher than the one observed by Singer et al. (37%) [79], and needs to be interpreted cautiously. It is well known though that MRI has higher-resolution capacity, and it has the ability to show small, focal abnormalities that cannot be seen on CT [1, 80]. It seems that a variety of antineoplastic agents have been implicated in the syndrome (Table 3). Although in the majority of the cases the medications have been administered intravenously it was interesting to find that intra-arterial [3], intraperitoneal [26] or hepatic transarterial chemoemblisation could also cause the syndrome [44, 62]. Platinum compounds, especially cisplatin, and gemcitabine, through the increased prothrombotic effect and endothelial dysfunction, were the most frequently encountered agents related with RPLS [81]. The fact that 70% of the cases presented the symptom in the initial period of the treatment may indicate that there is an inherent susceptibility of these patients to develop the syndrome.
The female prevalence of the syndrome has been observed in a previous review [82] as well as in a single institution cohort [79]. Although the exact mechanism underlying this observation is not clear, we can assume that estrogens may play a role. Estrogens have been implicated with endothelial dysfunction [83, 84] and can affect many vascular mediators, like endothelial nitric oxide synthase (eNOS), vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), transforming growth factor β (TGF-β), cyclooxygenases [83] regulating the vascular tone [85]. Nevertheless, the interplay between the offending anti-neoplastic agent, estrogens and endothelium in the development of the syndrome needs to be elucidated.
The treatment for RPLS must be symptomatic. Control of blood pressure is important especially for the patients who present with hypertension is commonly encountered with RPLS and in this review 86% of the cases had elevated BP during admission. Nevertheless it is increasingly noticed that normotensive patients may also present with the syndrome [30, 31, 39, 47, 54, 59, 77]. Treatment with anti-epileptics should be initiated in patients with seizures but there is no clear guideline about the overall duration of treatment. This need to be decided on individual basis and it is unlikely that long term antiepileptic treatment will be needed.
Withdrawal of the offending agent seems to fully or partially reverse the symptom or and the neuroimaging findings within a period of few days or weeks, respectively. In the majority of the cases there was no rechallenge of the patients with the same agent. In the few cases where the agent were reintroduced, including bevacizumab [38], no further RPLS-related symptoms were observed [38, 50, 66]. Nevertheless, when another patient was re-exposed to bevacizumab after the initial improvement of the symptoms, he experienced again similar symptoms and the drug was permanently discontinued [58]. In Singer et al. study, 7 out of the 17 patients (41%) that were rechallenged with the same anti-neoplastic treatment did not present the syndrome [79]. None of them though had bevacizumab rechallenge while this was a combined population involving patients with hematologic and solid malignancies. In general though, there is hesitance to carry on with the treatment with the offending agent unless there is strong clinical indication [50].
6. Conclusion
RPLS is a rare syndrome which is increasingly reported in the literature. The combination of the clinical symptoms and characteristic neuroimaging findings can help in the prompt diagnosis and management of this clinical entity. Clinicians need to have a high index of suspicion and request brain MRI when the syndrome is suspected.
References
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 334 (1996): 494-500.
- Sakai N, Kawasaki Y, Imaizumi T, et al. Two patients with focal segmental glomerulosclerosis complicated by cyclosporine-induced reversible posterior leukoencephalopathy syndrome. Clin Nephrol 73 (2010): 482-486.
- Ito Y, Arahata Y, Goto Y, et al. Cisplatin neurotoxicity presenting as reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol 19 (1998): 415-417.
- Rajasekhar A, George TJ, Jr. Gemcitabine-induced reversible posterior leukoencephalopathy syndrome (): a case report and review of the literature. Oncologist 12 (2007): 1332-1335.
- Khurana A, Dasanu CA. Posterior reversible encephalopathy syndrome due to targeted agents (): vemurafinib among suspects! J Oncol Pharm Pract 21 (2015): 443-450.
- Kimura T, Iio K, Imai E, et al. Exercise-induced acute kidney injury with reversible posterior leukoencephalopathy syndrome. Clin Exp Nephrol 14 (2010): 173-175.
- Aridon P, Ragonese P, Mazzola MA, et al. Reversible posterior leukoencephalopathy syndrome in a patient with thrombotic thrombocytopenic purpura. Neurol Sci 32 (2011): 469-472.
- Koehl B, Boyer O, Biebuyck-Gouge N, et al. Neurological involvement in a child with atypical hemolytic uremic syndrome. Pediatr Nephrol 25 (2010): 2539-2542.
- Eichler FS, Wang P, Wityk RJ, et al. Diffuse metabolic abnormalities in reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol 23 (2002): 833-837.
- Kamar N, Kany M, Bories P, et al. Reversible posterior leukoencephalopathy syndrome in hepatitis C virus-positive long-term hemodialysis patients. Am J Kidney Dis 37 (2001): E29.
- Fujieda Y, Kataoka H, Odani T, et al. Clinical features of reversible posterior leukoencephalopathy syndrome in patients with systemic lupus erythematosus. Mod Rheumatol 21 (2011): 276-281.
- Min L, Zwerling J, Ocava LC, et al. Reversible posterior leukoencephalopathy in connective tissue diseases. Semin Arthritis Rheum 35 (2006): 388-395.
- Irvin W, MacDonald G, Smith JK, Kim WY. Dexamethasone-induced posterior reversible encephalopathy syndrome. J Clin Oncol 25 (2007): 2484-2486.
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345 (2001): 725-730.
- Russell MT, Nassif AS, Cacayorin ED, et al. Gemcitabine-associated posterior reversible encephalopathy syndrome (): MR imaging and MR spectroscopy findings. Magn Reson Imaging 19 (2001): 129-132.
- Sueblinvong T, Noophun P, Pataradool K, et al. Posterior leukoencephalopathy following cisplatin, bleomycin and vinblastine therapy for germ cell tumor of the ovary. J Obstet Gynaecol Res 28 (2002): 99-103.
- Ozcan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med 354 (2006): 980-982
- Allen JA, Adlakha A, Bergethon PR. Reversible posterior leukoencephalopathy syndrome after bevacizumab/FOLFIRI regimen for metastatic colon cancer. Arch Neurol 63 (2006): 1475-1478.
- Manchana T, Sirisabya N, Lertkhachonsuk R, Tresukosol D. Transient cortical blindness during chemotherapy (PVB) for ovarian germ cell tumor. J Med Assoc Thai 89 (2006): 1265-1268.
- Skelton MR, Goldberg RM, O'Neil BH. A case of oxaliplatin-related posterior reversible encephalopathy syndrome. Clin Colorectal Cancer 6 (2007): 386-388.
- Moris G, Ribacoba R, Gonzalez C. Delayed posterior encephalopathy syndrome following chemotherapy with oxaliplatin and gemcitabine. J Neurol 254 (2007): 534-535.
- Pinedo DM, Shah-Khan F, Shah PC. Reversible posterior leukoencephalopathy syndrome associated with oxaliplatin. J Clin Oncol 25 (2007): 5320-5321.
- Connolly RM, Doherty CP, Beddy P, O'Byrne K. Chemotherapy induced reversible posterior leukoencephalopathy syndrome. Lung Cancer 56 (2007): 459-463.
- Vieillot S, Pouessel D, de Champfleur NM, et al. Reversible posterior leukoencephalopathy syndrome after carboplatin therapy. Ann Oncol 18 (2007): 608-609.
- Peter S, Hausmann N, Schuster A, Boehm HF. Reversible posterior leukoencephalopathy syndrome and intravenous bevacizumab. Clin Experiment Ophthalmol 36 (2008): 94-96.
- Onujiogu N, Lengyel E, Yamada SD. Reversible posterior leukoencephalopathy syndrome following intravenous paclitaxel and intraperitoneal cisplatin chemotherapy for fallopian tube cancer. Gynecol Oncol 111 (2008): 537-539.
- Burki F, Badie K, Bartoli P, et al. Reversible posterior leukoencephalopathy syndrome associated with bevacizumab/doxorubicin regimen. Br J Clin Pharmacol 65 (2008): 793-794.
- El Maalouf G, Mitry E, Lacout A, et al. Isolated brainstem involvement in posterior reversible leukoencephalopathy induced by bevacizumab. J Neurol 255 (2008): 295-296.
- Sharief U, Perry DJ. Delayed reversible posterior encephalopathy syndrome following chemotherapy with oxaliplatin. Clin Colorectal Cancer 8 (2009): 163-165.
- Nguyen MT, Virk IY, Chew L, Villano JL. Extended use dexamethasone-associated posterior reversible encephalopathy syndrome with cisplatin-based chemotherapy. Journal of Clinical Neuroscience 16 (2009): 1688-1690.
- Bhatt A, Farooq MU, Majid A, Kassab M. Chemotherapy-related posterior reversible leukoencephalopathy syndrome. Nat Clin Pract Neurol 5 (2009): 163-169.
- Kwon EJ, Kim SW, Kim KK, et al. A case of gemcitabine and cisplatin associated posterior reversible encephalopathy syndrome. Cancer Res Treat 41 (2009): 53-55.
- Chue AL, Fernando IN, Hussain SA, Yates DA. Chemotherapy related encephalopathy in a patient with Stage IV cervical carcinoma treated with cisplatin and 5-fluorouracil: a case report. Cases J 2 (2009): 8526.
- Kim CH, Kim CH, Chung CK, Jahng TA. Unexpected seizure attack in a patient with spinal metastasis diagnosed as posterior reversible encephalopathy syndrome. J Korean Neurosurg Soc 50 (2011): 60-63.
- Han CH, Findlay MP. Chemotherapy-induced reversible posterior leucoencephalopathy syndrome. Intern Med J 40 (2010): 153-159.
- Maeda T, Kikuchi E, Matsumoto K, et al. Gemcitabine and cisplatin chemotherapy induced reversible posterior leukoencephalopathy syndrome in a bladder cancer patient. Int J Clin Oncol 15 (2010): 508-511.
- Lau PC, Paunipagar B. Posterior reversible encephalopathy syndrome with bevacizumab. Hong Kong Med J 17 (2011): 80-81.
- Lou E, Turner S, Sumrall A, et al. Bevacizumab-induced reversible posterior leukoencephalopathy syndrome and successful retreatment in a patient with glioblastoma. J Clin Oncol 29 (2011): e739-742.
- Lewis-Hanna DL, Pamma G. Diagnostic uncertainty around seizures in advanced malignancy. BMJ Case Rep (2011).
- Marrone LC, Marrone BF, de la Puerta Raya J, et al. Gemcitabine monotherapy associated with posterior reversible encephalopathy syndrome. Case Rep Oncol 4 (2011): 82-87.
- Helissey C, Chargari C, Lahutte M, et al. First case of posterior reversible encephalopathy syndrome associated with vinflunine. Invest New Drugs 30 (2012): 2032-2034.
- Cioffi P, Laudadio L, Nuzzo A, et al. Gemcitabine-induced posterior reversible encephalopathy syndrome (): a case report. J Oncol Pharm Pract 18 (2012): 299-302.
- Femia G, Hardy TA, Spies JM, Horvath LG. Posterior reversible encephalopathy syndrome following chemotherapy with oxaliplatin and a fluoropyrimidine (): a case report and literature review. Asia Pac J Clin Oncol 8 (2012): 115-122.
- Pawar PS, Noviawaty I, Zaidat OO. Unusual case of intra-arterial doxorubicin chemoembolization-associated posterior reversible encephalopathy syndrome. Neurologist 18 (2012): 49-50.
- Kaneda H, Okamoto I, Satoh T, Nakagawa K. Reversible posterior leukoencephalopathy syndrome and trastuzumab. Invest New Drugs 30 (2012): 1766-1767.
- Chang Y, Mbeo G, Littman SJ. Reversible posterior leukoencephalopathy syndrome associated with concurrent bevacizumab, gemcitabine, and oxaliplatin for cholangiocarcinoma. J Gastrointest Cancer 43 (2012): 505-507.
- Ryan SA, Maceneaney P, O'Reilly SP, et al. Reversible posterior leukoencephalopathy induced by carboplatin and etoposide. Med Oncol 29 (2012): 1287-1291.
- Imai H, Okuno N, Ishihara S, et al. Reversible posterior leukoencephalopathy syndrome after carboplatin and paclitaxel regimen for lung cancer. Intern Med 51 (2012): 911-915.
- Chen YH, Huang CH. Reversible posterior leukoencephalopathy syndrome induced by vinorelbine. Clin Breast Cancer 12 (2012): 222-225.
- Zahir MN, Masood N, Shabbir-Moosajee M. Cisplatin-induced posterior reversible encephalopathy syndrome and successful re-treatment in a patient with non-seminomatous germ cell tumor (): a case report. J Med Case Rep 6 (2012): 409.
- Seet RC, Rabinstein AA. Clinical features and outcomes of posterior reversible encephalopathy syndrome following bevacizumab treatment. QJM 105 (2012): 69-75.
- Sclafani F, Giuseppe G, Mezynksi J, et al. Reversible posterior leukoencephalopathy syndrome and bevacizumab in breast cancer. J Clin Oncol 30 (2012): e257-259.
- Truman N, Nethercott D. Posterior reversible encephalopathy syndrome (PRES) after treatment with oxaliplatin and 5-fluorouracil. Clin Colorectal Cancer 12 (2013): 70-72.
- Endo A, Yoshida Y, Nakashima R, et al. Capecitabine induces both cardiomyopathy and multifocal cerebral leukoencephalopathy. Int Heart J 54 (2013): 417-420.
- Abbas O, Shamseddin A, Temraz S, Haydar A. Posterior reversible encephalopathy syndrome after bevacizumab therapy in a normotensive patient. BMJ Case Rep (2013).
- Dersch R, Stich O, Goller K, et al. Atypical posterior reversible encephalopathy syndrome associated with chemotherapy with Bevacizumab, Gemcitabine and Cisplatin. J Neurol 260 (2013): 1406-1407.
- Porcello Marrone LC, Marrone BF, Pascoal TA, et al. Posterior Reversible Encephalopathy Syndrome Associated with FOLFOX Chemotherapy. Case Rep Oncol Med (2013): 306983.
- Goto N, Mimura J. Gastrointestinal bevacizumab-induced reversible posterior leukoencephalopathy syndrome in patient with rectal cancer. J Gastroenterol Hepatol 29 (2014): 895.
- Wang W, Zhao LR, Lin XQ, Feng F. Reversible posterior leukoencephalopathy syndrome induced by bevacizumab plus chemotherapy in colorectal cancer. World J Gastroenterol 20 (2014): 6691-6697.
- Chen H, Modiano MR, Neal JW, et al. A phase II multicentre study of ziv-aflibercept in combination with cisplatin and pemetrexed in patients with previously untreated advanced/metastatic non-squamous non-small cell lung cancer. Br J Cancer 110 (2014): 602-608.
- Dedic Plavetic N, Rakusic Z, Ozretic D, et al. Fatal outcome of posterior "reversible" encephalopathy syndrome in metastatic colorectal carcinoma after irinotecan and fluoropyrimidine chemotherapy regimen. World J Surg Oncol 12 (2014): 264.
- Kistler CA, McCall JC, Ghumman SS, et al. Posterior reversible leukoencephalopathy syndrome secondary to hepatic transarterial chemoembolization with doxorubicin drug eluting beads. J Gastrointest Oncol 5 (2014): E43-45.
- Fitzgerald RT, Wright SM, Samant RS, et al. Elevation of serum lactate dehydrogenase at posterior reversible encephalopathy syndrome onset in chemotherapy-treated cancer patients. J Clin Neurosci 21 (2014): 1575-1578.
- Tang KH. Oxaliplatin-induced posterior reversible encephalopathy syndrome with isolated involvement of pons. J Cancer Res Ther 11 (2015): 1022.
- Ladwa R, Peters G, Bigby K, Chern B. Posterior Reversible Encephalopathy Syndrome in Early-Stage Breast Cancer. Breast J 21 (2015): 674-677.
- Kandemir M, Kucukkaya B, Tepe MS, et al. Reversible Posterior Leukoencephalopathy Syndrome Due to Carboplatin and Paclitaxel Therapy. Balkan Med J 32 (2015): 421-425.
- Xie C, Jones VT. Reversible posterior leukoencephalopathy syndrome following combinatorial cisplatin and pemetrexed therapy for lung cancer in a normotensive patient: A case report and literature review. Oncol Lett 11 (2016): 1512-1516.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults (): report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311 (2014): 507-520.
- Vaughn C, Zhang L, Schiff D. Reversible posterior leukoencephalopathy syndrome in cancer. Curr Oncol Rep 10 (2008): 86-91.
- Icli F, Karaoguz H, Dincol D, et al. Severe Vascular Toxicity Associated with Cisplatin-Based Chemotherapy. Cancer 72 (1993): 587-593.
- Izumi Y, Xu L, di Tomaso E, et al. Tumour biology (): herceptin acts as an anti-angiogenic cocktail. Nature 416 (2002): 279-280.
- Oppenheim C, Galanaud D, Samson Y, et al. Can diffusion weighted magnetic resonance imaging help differentiate stroke from stroke-like events in MELAS? J Neurol Neurosurg Psychiatry 69 (2000): 248-250.
- Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol 21 (2000): 1199-1206.
- Myint ZW, Sen JM, Watts NL, et al. Reversible posterior leukoencephalopathy syndrome during regorafenib treatment (): a case report and literature review of reversible posterior leukoencephalopathy syndrome associated with multikinase inhibitors. Clin Colorectal Cancer 13 (2014): 127-130.
- Larsen FO, Hansen SW. Severe neurotoxicity caused by gemcitabine treatment. Acta Oncol 43 (2004): 590-591.
- Rahal AK, Truong PV, Kallail KJ. Oxaliplatin-Induced Tonic-Clonic Seizures. Case Rep Oncol Med (2015): 879217.
- Ki SS, Jeong JM, Kim SH, et al. A case of neurotoxicity following 5-fluorouracil-based chemotherapy. Korean J Intern Med 17 (2002): 73-77.
- Henderson RD, Rajah T, Nicol AJ, Read SJ. Posterior leukoencephalopathy following intrathecal chemotherapy with MRA-documented vasospasm. Neurology 60 (2003): 326-328.
- Singer S, Grommes C, Reiner AS, et al. Posterior Reversible Encephalopathy Syndrome in Patients With Cancer. Oncologist 20 (2015): 806-811.
- Schwartz RB, Jones KM, Kalina P, et al. Hypertensive encephalopathy: findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol 159 (1992): 379-383.
- Dasanu CA. Gemcitabine: vascular toxicity and prothrombotic potential. Expert Opin Drug Saf 2008 7 (): 703-716.
- Marinella MA, Markert RJ. Reversible posterior leucoencephalopathy syndrome associated with anticancer drugs. Intern Med J 39 (2009): 826-834.
- Abdu TA, Elhadd T, Pfeifer M, Clayton RN. Endothelial dysfunction in endocrine disease. Trends Endocrinol Metab 12 (2001): 257-265.
- Lee SJ, Lee DW, Kim KS, Lee IK. Effect of estrogen on endothelial dysfunction in postmenopausal women with diabetes. Diabetes Res Clin Pract 2 (2001): S81-92.
- Hermenegildo C, Oviedo PJ, Cano A. Cyclooxygenases regulation by estradiol on endothelium. Curr Pharm Des 12 (2006): 205-215.
