Protective Effect of Withania Somnifera Bleomycin Induced Pulmonary Fibrosis in Experimental Rats
Article Information
Maaz Naqvi1, Mohd Rafi Reshi1, Muzammil Muzaffar2, Saman Anees3, Arunabha Ray1, *
1Department of Pharmacology, Hamdard Institute of Medical Sciences & Research, Jamia Hamdard, New Delhi, India
2Department of Physiology, Hamdard Institute of Medical Sciences & Research, Jamia Hamdard, New Delhi, India
3Department of Amraze Niswan Wa Atfal, SUMER, Jamia Hamdard, New Delhi, India
*Corresponding author: Prof. Arunabha Ray, Head Department of Pharmacology, Hamdard Institute of Medical Sciences & Research, Jamia Hamdard, New Delhi, India
Received: 22 November 2022; Accepted: 05 December 2022; Published: 14 December 2022
Citation: Maaz Naqvi, Mohd Rafi Reshi, Muzammil Muzaffar, Saman Anees, Arunabha Ray. Protective Effect of Withania Somnifera Bleomycin Induced Pulmonary Fibrosis in Experimental Rats. Journal of Pharmacy and Pharmacology Research 6 (2022): 186-191.
View / Download Pdf Share at FacebookAbstract
Background: A lung condition that progresses fatally and has a high mortality rate is pulmonary fibrosis. One of the most often utilised chemotherapeutic medicines for treating various carcinomas is bleomycin (BLM). BLM's pulmonary toxicity is its most serious side effect, hence it has frequently been reported to be among the most commonly utilised drugs for inducing experimental lung fibrosis.
Methods: Bleomycin was given to rats once (on day 0) in order to cause lung fibrosis. By observing variations in cytokines and markers of oxidative stress in comparison to that in normal control rats, the lung fibrosis model was confirmed. In the aforementioned rat lung fibrosis model, the effects of Withania somnifera were examined on cytokine and oxidative marker levels.
Results: The efficacy of Withania somnifera to lessen BLM-induced lung fibrosis has been examined in the current investigation. For four weeks, BLM was supplied intratracheally, while Withania somnifera was given orally in doses of 200 and 400 mg/kg. In addition to considerably lowering tissue homogenate lung MDA and raising lung GSH, Withania somnifera also significantly reduced BALF's and serum TGF-1 and IL 13 levels.
Conclusion: Withania somnifera can be suggested as a viable therapeutic agent for the management of idiopathic pulmonary fibrosis against lung fibrosis generated by bleomycin in rats, according to the data, and the effects were comparable to those seen with standard treatment.
Keywords
Fibrosis, Withania somnifera, Biomarkers, Lung
Fibrosis articles, Withania somnifera articles, Biomarkers articles, Lung articles
Article Details
1. Introduction
Idiopathic pulmonary fibrosis is a rare lung condition with no known origin that results in an immediate demise [1]. Epidemiological studies show that over the past two to three decades, the incidence of IPF has been steadily rising [2]. Two anti-fibrotic drugs, pirfenidone and nintedanib, have recently been found to be effective in slowing disease progression and have been licenced as treatments, despite the fact that the aetiology and pathophysiology of IPF are still unknown [3, 4]. Clinical management of IPF is still difficult due to the lack of specific indicators of [5].
A fatal lung condition that progresses over time is pulmonary fibrosis. It is the final stage of a variety of inflammatory lung diseases. The major characteristics of pulmonary fibrosis are loss of alveolar structure, accretion of myofibroblasts, modification of the lung parenchyma, and excessive extracellular matrix depositions [6]. With mean survival duration of roughly 3 years, pulmonary fibrosis is one of the most prevalent interstitial lung disorders, affecting over 5 million people globally [7]. Bleomycin is one anti-neoplastic medication that can cause pulmonary fibrosis as a side effect (BLM). Additionally, smoking cigarettes and breathing mineral dusts or asbestos are pathogenesis-related variables [8].
According to reports, BLM-induced pulmonary fibrosis in rats and mice can be used to examine the mechanisms behind the advancement of human pulmonary fibrosis and the effects of different medications. BLM causes the production of reactive oxygen species (ROS), which attach to DNA and cause DNA damage, which is thought to start an inflammatory and fibro-proliferative response. Additionally, BLM is said to encourage the loss of endogenous antioxidant defences, aggravating tissue damage caused by oxidants. [9].
The Indian traditional medical system known as Ayurveda dates back to 6000 BC (Charak Samhita, 1949). Ashwagandha (Withania somnifera) has been utilised as a Rasayana throughout the majority of these 6000 years. The root of Ashwagandha is valued for its tonic, narcotic, diuretic, anthelmintic, astringent, stimulant, and thermogenic properties. It possesses a variety of medicinal properties, including those that are anti-inflammatory, analgesic, anti-tumor, antioxidant, immunomodulatory, and others [10, 11]. The objective of the current study protocol was to assess Withania somnifera's potential to reduce BLM-induced lung fibrosis in a rat model.
2. Materials & Methods
2.1 Drugs and Chemicals
The drug and chemicals are taken from different suppliers like Withania sominefera provided by Natural Remedies Bengaluru, Bleomycin and Pirfenidone was purchased from Cipla Ltd., and ketamine from pharmacy shop. Other chemicals were taken from SRL, New Delhi. Elisa kits were purchased from Elabscience.
2.2 Animals
The study used both sex Wistar rats (180-220 g). Animals were taken from the Central Animal House Facility, Hamdard University and kept in a controlled environment. They were provided with food and drink. Animals were cared for according to CPCSEA criteria for animal usage, which were approved by the Institutional Animal Ethics Committee (IAEC) protocol number 1444 (Registration number 173/GO/ReBi/S/2000/CPCSEA).
2.3 Bleomycin induced lung fibrosis in rats
In this model animals are randomly divided into 5 groups of 6 rats each. Group I served as normal control. Group-II experimental control was given single dose of bleomycin (intratracheal injection 1.5 U in 0.3ml saline) zero day [12]. Group-III and Group-IV given Withania somnifera (WS) at two different doses (200 and 400 mg/kg p.o.,) started next day after bleomycin. Group-V was given as standard (pirfenidone dose 10mg/kg) started same on first day. After 28days, blood samples were collected from all animals by retro-orbital under mild anesthesia, after those animals have been sacrificed. The blood samples analysed for lung fibrosis markers. Lungs were harvested, rinsed in ice-cold saline. The left lobes from all the lungs were isolated for preparation of lung homogenate.
2.4 Collection of bronchoalveolar lavage fluid (BALF)
The tracheas were exposed, cannulated, and the thoracic cavity was opened. 3 times, 2 ml of sterile 0.9% saline was slowly infused into the lungs. After gently squeezing the chest numerous times, 50-70% of the recovery was obtained. The BALF was centrifuged using a cooled centrifuge at 2000 rpm; 4°C for 10 min. Supernatant was then isolated and placed in a deep freezer for various tests.
2.5 Assessment of lung interleukin-13 (IL-13) and TGF-β 1
Lung content of IL-13 and TGF-β 1 was quantified using commercially available enzyme-linked immunosorbent assay (ELISA) kit, according to the ELISA manual instructions.
2.6 Estimation of MDA levels
Malondialdehyde (MDA) is widely used as oxidative stress biomarker in biomedical research. Lipid peroxidation is measured spectrophotometrically as 2-thiobarbituric acid-reactive substance (TBARS) in supernatant of liver homogenate. 0.1 ml of supernatant was mixed with 0.2 ml of sodium dodecyl sulfate (8.1 %), 1.5 ml of 20 % acetic acid and 1.5 ml of 2-thiobarbituric acid (0.8 %). The reaction mixture was finally made up to 4.0 ml with distilled water. After vortexing, samples were incubated for 1 h in 95o C and after cooling with tap water; 1.0 ml of distilled water and 5.0 ml of mixture of butanol-pyridine 15:1 (v/v) were added. The mixture was shaken for 10 min. and then centrifuged at 4000 rpm for 10 min. Then Butanol-pyridine layer was taken and measured spectrophotometrically at 532 nm. TBARS values are expressed as MDA equivalents. 1, 1, 3, 3-tetramethoxypropane (TMP) was used as the standard [13]. Protein estimation by lowry method.
2.7 Assay of reduced glutathione (GSH)
Glutathione (GSH) levels were estimated by the method of Ellman [13]. This assay is based on the enzymatic recycling procedure in which glutathione was sequentially oxidized by the DTNB and reduced by NADPH in the presence of glutathione reductase. For assay, an equal quantity of sample was mixed with 10% trichloroacetic acid and centrifuged to separate the proteins. To 0.1 ml of this supernatant, 2 ml of phosphate buffer (pH 8.4), 0.5 ml of 5’5-dithiobis (2-nitrobenzoic acid) and 0.4 ml of double distilled water was added. The mixture was vortexes and absorbance was read at 412 nm within 15 min. The concentration of 2-nitro-5-benzoic acid formation was measured and reduced glutathione is expressed as µmol/mg protein.
2.8 Statistical Analysis
The values were expressed as mean ± standard error of the mean. One-way analysis of variance (ANOVA) followed by appropriate post hoc test (Tukey test) were used for analysis. p< 0.05 was considered as statistically significant.
3. Results
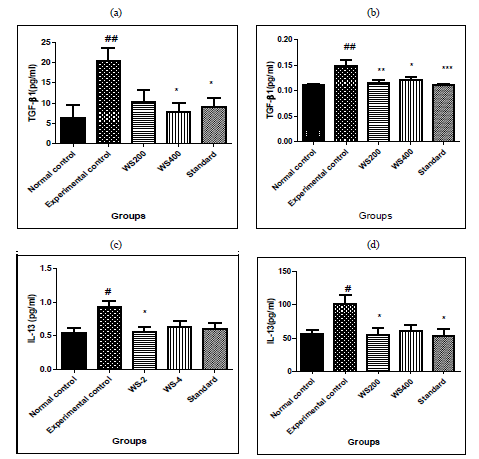
3.1 Effects of Withania somnifera on cytokine (TGF-β1) and IL-13 in bleomycin induced lung fibrosis in rats
In experimental control, bleomycin given single dose resulted in significant increase in levels of TGF-β1 both in serum and BALF (p<0.01) as compared to that in normal control group. Similarly, in Group-III and IV treatment with two different doses of withania somnifera (200 and 400mg/kg) attenuated the effect as it reduced significantly the levels of serum TGF-β1 (p < 0.05 at 400mg/kg dose), in BALF it reduced significantly both the doses of WS (p < 0.01 at 200mg/kg and p< 0.05 at 400mg/kg) as compared to that in Experimental control. Pre-treatment with standard also significant attenuated the effects of bleomycin and reduced the levels of serum TGF-β1 (p< 0.05) and in BALF (p< 0.001). In IL-13, bleomycin given single dose resulted in significant increase in levels of IL-13 both in serum and BALF (p<0.05) as compared to that in normal control group.
Similarly, in Group-III and IV treatment with two different doses of withania somnifera (200 and 400mg/kg) attenuated the effect as it reduced significantly the levels of serum IL-13 and BALF (p < 0.05 at 200mg/kg dose), as compared to that in Experimental control. Pre-treatment with standard also significant attenuated the effects of bleomycin and reduced the levels of serum IL-13 (p< 0.05) and in BALF no statistical difference. The results are shon in [Figure 1]
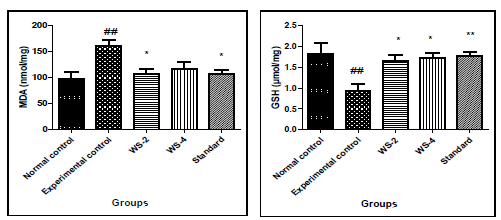
3.2 Effects of Withania somnifera on oxidative stress parameters in bleomycin induced lung fibrosis
In experimental control, bleomycin given single dose resulted in significant increase in levels of MDA in tissue homgenate (p<0.01) and decrease in the level of GSH (p<0.01) as compared to that in normal control group. Similarly, in Group-III and IV treatment with two different doses of withania somnifera (200 and 400mg/kg) attenuated the effect as it reduced significantly the levels of MDA (p<0.05 at 200mg/kg dose) and increased the level of GSH significantly both the doses of WS (p< 0.05 at 400mg/kg) as compared to that in Experimental control. Pre-treatment with standard also significant attenuated the effects of bleomycin and reduced the levels of MDA (p< 0.05) and increased GSH (p< 0.01).The results are shown in [Figure 2].
4. Discussion
Increased pulmonary myofibroblasts, loss of alveolar cells, extracellular matrix build up, and lung stiffness are the hallmarks of pulmonary fibrosis. It has long been known that the cytokine TGF-β plays a significant role in the development of lung fibrosis by encouraging fibroblast differentiation into myofibroblasts. Surprisingly, our recent work found that miR-133a is induced by TGF-β1 and functions as an anti-fibrotic agent. By focusing on a group of TGF-β1 signalling pathway-related proteins in human pulmonary fibroblasts, it acts as a negative feedback regulator of profibrogenic pathways. We used RNA-sequencing to discover the miRNAs that were differentially expressed in HFL cells with or without TGF-β1 treatment since TGF-β promotes the differentiation of fibroblast cells into myofibroblast cells, which is a key factor in pulmonary fibrosis. Our first theory was that TGF-β1 profibrogenic signalling may be mediated by miRNAs up-regulated by TGF-1 [14, 15].
Our results demonstrate that miR-133a levels are higher in differentiated myofibroblasts produced by TGF-β1 and correlate with indicators of pulmonary fibrosis in a pre-defined collection of miRNAs relevant to pulmonary disease. The second cytokine TNF-α, which is similarly secreted by cells during wound healing, did not reproduce this elevation of miR133a by TGF-β1, which is time- and concentration-dependent. Inhibitors of the Smad3 and p38-MAPK signalling pathways inhibited the TGF-β1-induced up-regulation of miR-133a, indicating that miR-133a may act as a feedback mediator down-regulating profibrotic genes and desensitising TGF-β1 signalling pathways. In fact, miR-133a mimics decreased TGF-β1 induced -SMA expression in these fibroblasts, while miR-133a inhibitor increased -SMA expression. Interestingly, miR-133a does not affect the extracellular matrix degradation-promoting microenvironment circumstances that TGF-β1-induced MMP-2 or MMP-9 gene expression or enzyme activity [16]. Chronic pulmonary overexpression of IL-13 causes parenchymal and airway tissue fibrosis, to investigate the mechanism(s) by which IL-13 promotes tissue fibrosis. This approach allowed us to investigate the mechanism(s) by which IL-13 causes tissue fibrosis [17, 18].
The current study provides information on Withania somnifera's capacity to protect the lungs from BLM-induced pulmonary fibrosis. A well-established rodent model using a single intratracheal BLM injection was modified for the current study. Biochemical dynamics were markedly hampered by BLM instillation, which also had an impact on pulmonary physiological processes. According to theory, BLM injection causes oxidative stress and inflammation, which feed off of each other and simultaneously stop the injury/repair process, causing lung fibrosis. Bleomycin (BLM) is thought to produce ROS [19] that attack bio-macromolecules such DNA, protein, and lipid, leading to lipid peroxidation that causes biochemical and physiological dysfunctions [20].
According to Daniil Papageorgiou, oxidative stress is a major factor in the pathological development of pulmonary fibrosis. According to certain reports, the aetiology of fibrosis may be at least partially attributed to the production of ROS and reactive nitrogen species [28,29]. Patients with pulmonary fibrosis have lungs that contain oxidative stress markers, and in animal models, abnormal antioxidant activity increased pulmonary fibrosis [21]. The current investigation found that intratracheal BLM instillation seriously jeopardised the balance of oxidants and antioxidants in the body, as evidenced by the considerable increase in lung MDA content and the concurrent decrease in GSH content [22]. The antioxidant effect of Withania somnifera was confirmed by the considerable protection it provided against BLM-induced impairment in oxidants/antioxidants hemostasis. The current investigation found that intratracheal instillation of BLM generated persistent lung inflammation, which was supported by biochemical evaluations, as one of the other causes of BLM-induced pulmonary injury. Following the use of Withania somnifera, all of these symptoms improved.
Cytokine level TGF-β1 and IL-13 increased in bleomycin induced experimental group both in serum and BALF. After the treatment of Withania somnifera they reduced the level significantly both serum and BALF as well as in standard treatment. Lipid peroxidation, a consequence of oxidative stress, was quantified by estimating MDA levels in lung homogenate. MDA levels in the experimental group were markedly increased by bleomycin. This increase was very significant when compared to the usual control group that did not receive bleomycin. By-products of metabolism that are extremely reactive might have accelerated lipid peroxidation. By significantly reducing the amounts of MDA in the treatment group's lung homogenate, Withania somnifera was effective in preventing lipid peroxidation in comparison to the experimental control group. Lower levels of MDA in the lung homogenate of the treatment group indicate that Withania somnifera plays a strong protective function against bleomycin-induced lipid peroxidation.
According to the current research, withania somnifera significantly decreased serum and BALF levels of IL-13 and TGF-β1 when administered. Furthermore, Withania somnifera protected against elevated reactive oxygen levels in response to bleomycin, as shown by decreased MDA and noticeably higher GSH levels, which were measured using oxidative stress measures in lung homogenates. These results demonstrated that withania somnifera is a protective drug that prevents pulmonary lung fibrosis.
5. Conclusion
Withania somnifera confers significant protective effect against BLM-induced pulmonary fibrosis. Combined antioxidant and anti-fibrotic effects are believed to be implicated in the observed efficacy.
Acknowledgement
The research was supported by grants from the ICMR, New Delhi, which is duly acknowledged.
Authors Contributions
Mohd Rafi Reshi and Maaz Naqvi were involved in the conduct of experiments, acquisition, and analysis of data and drafting of the manuscript. Muzammil Muzaffar also conducting experiment, dosing and drafting of manuscript. Saman Anees was also involved in experiment, Drafting and analysis of the study. Arunabha Ray was involved in planning of the study, interpretation of data and critical reviewing of manuscript. All authors approved the final version of the manuscript.
Conflict of interest
No conflict of interest
Ethical statement
Ethical approval was required as this study involve laboratory animals.
Reference
- Julien G, Catherine M, Monique He, Jean-Louis C, Renaud L. Blood biomarkers in Idiopathic Pulmonary Fibrosis. Lung 195 (2017): 273-280.
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary febrosis. Am J Respir Crit Care Med 174 (2006): 810-816.
- King TE, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary febrosis. N Engl J Med 370 (2014): 2083-2092.
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efcacy and safety of nintedanib in idiopathic pulmonary febrosis. N Engl J Med 370 (2014): 2071-2082.
- Maher TM. Profil Eing idiopathic pulmonary febrosis: rethinking biomarker discovery. Eur Respir Rev 22 (2013): 148-152.
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. New Engl J Med 345 (2001): 517-525.
- Cottin V. Changing the idiopathic pulmonary fibrosis treatment approach and improving patient outcomes. Eur Respirat Rev 21 (2012): 161-167
- Green FH. Overview of pulmonary fibrosis. Chest 122 (2002): 334S-9S.
- Atzori L, Chua F, Dunsmore SE, Willis D, Barbarisi M, McAnulty RJ, et al. Attenuation of bleomycin induced pulmonary fibrosis in mice using the heme oxygenase inhibitor Zn-deuteroporphyrin IX-2,4-bisethylene glycol. Thorax 59 (2004): 217-23.
- Narendra S, Mohit B, Prashanti de-J, Marilena G. An overview on Ashwagandha: a rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med.8 (2011): 208-213.
- Sumaira S, Gulzar M, Muhammad AH, Muhammad A, Syed Nasir AB. Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran J Basic Med Sci, 3 (2020).
- Marwa SZ, Ramy Ahmed A-S, Eman S, Ghada MS, Hatem Abdel-Rahman S. Attenuation of Bleomycin-induced pulmonary fibrosis in rats by flavocoxid treatment. Egyptian Journal of Basic and Applied Sciences 4 (2017): 256.
- Mohd RR, Kavita G, Maaz N, Nafaa H, Arunabha R. Hepatoprotective Effects of Dawa-Ul-Kurkum, a Unani Polyherbal Preparation and the Possible Mechanisms in Experimental Model of Ethanol Induced Liver Damage in Rats. Journal of Pharmacy and Pharmacology Research 6 (2022): 122-130.
- Yang, S. MiR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J. 27 (2013): 2382-2391.
- Huang Y, He Y, Li J. MicroRNA-21: a central regulator of fibrotic diseases via various targets. Curr. Pharm. Des. 21 (2015): 2236-2242.
- Peng W, Yan X, Peter WA, Yapei H, Qin M, Linghai L., et al. Transforming growth factor (TGF)-β1-induced miR-133a inhibits myofibroblast differentiation and pulmonary fibrosis. Cell Death and Disease 10 (2019).
- Clark DA, Coker R. Transforming growth factor- (TGF-). Int. J. Biochem. Cell Biol. 30 (1998): 293-298.
- Shah M, Revis D, Herrick S, Baillie R, Thorgeirson S, Ferguson M, et al. Role of elevated plasma transforming growth factor-1 levels in wound healing. Am. J. Pathol. 154 (1999):1115-1124.
- Chitra P, Saiprasad G, Manikandan R, Sudhandiran G. Berberine attenuates bleomycin induced pulmonary toxicity and fibrosis via suppressing NF-jB dependant TGF-b activation: a biphasic experimental study. Toxicol Lett 219 (2013): 178-93.
- Liang X, Tian Q, Wei Z, Liu F, Chen J, Zhao Y, et al. Effect of Feining on bleomycin-induced pulmonary injuries in rats. J Ethnopharmacol 134 (2011): 971-976.
- Daniil ZD, Papageorgiou E, Koutsokera A, Kostikas K, Kiropoulos T, Papaioannou AI, et al. Serum levels of oxidative stress as a marker of disease severity in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther 21(2008): 26-31.
- Sogut S, Ozyurt H, Armutcu F, Kart L, Iraz M, Akyol O, et al. Erdosteine prevents bleomycin-induced pulmonary fibrosis in rats. Eur J Pharmacol 494 (2004): 213-220.