Prevalence and Risk Factors of Hypertensive Disorders in Pregnancy: Case of Mezam Division, NWR Cameroon
Article Information
Nkem Ernest Njukang1,5*, Thomas Obinchemti EGBE2, Martin Sama1, Tah Aldof Yoah1, Joseph Kamgno3, 4
1Department of Public Health and Hygiene, Global Health System Solutions (GHSS), Yaounde, Cameroon
2Department of Obstetrics and Gynaecology, Faculty of Health Sciences, University of Buea, Buea, Cameroon
3Faculty of Medicine and Biomedical Sciences, University of Yaounde I, Yaounde, Cameroon
4Centre for Research on Filariasis and other Tropical Diseases (CRFilMT), Yaounde, Cameroon
5Quality Assurance/Quality Management System Mentor, Global Health System Solutions (GHSS), Yaounde, Cameroon
*Corresponding author: Nkem Ernest Njukang, Department of Public Health and Hygiene, Quality Assurance/Quality Management System Mentor, Global Health System Solutions (GHSS), Yaounde, Cameroon
Received: 29 July 2020; Accepted: 07 August 2020; Published: 13 August 2020
Citation:
Nkem Ernest Njukang, Thomas Obinchemti EGBE, Martin Sama, Tah Aldof Yoah, Joseph Kamgno. Prevalence and Risk Factors of Hypertensive Disorders in Pregnancy: Case of Mezam Division, NWR Cameroon. Journal of Women’s Health and Development 3 (2020): 247-267.
View / Download Pdf Share at FacebookAbstract
Background: Hypertensive disorders of pregnancy (HDP) are a global public health concern both in developed and developing countries. Evidence regarding the risk factors of HDP are limited in Cameroon. The aim of the study was to determine the prevalence and risk factors of HDP in Mezam Division.
Methods: A cross sectional study conducted between April-July, 2018 in Mezam Division. Consecutive sampling was used to recruit 1210 pregnant women. Descriptive statistics, chi-square (χ2) test and multivariate logistic regression were used for analysis.
Results: The prevalence of HDP was 14.5%; of which 3.4% chronic HTN (CH), 31.8% gestational HTN (GH), 48.3% preeclampsia (PE), 5.7% PE superimpose on CH and 10.8% severe PE. Risk factors of CH were: Family history HTN [Adjusted Odd Ratio (AOR), 95%Confidence Interval (CI): 3.70 (1.7-11.6)], stress [2.80 (1.1-10.9)] and overweight/obese [2.6 (1.3-6.7)]. For GH, Single/separated [2.7 (1.5-4.7)], family history [4.4 (1.5-13.1)], alcohol [2.8 (1.1-5.4)] and previous history of HDP [4.9 (1.5-10.2)] were significant. For PE, overweight/obese [2.7 (1.4-7.1)], previous history of HDP [3.5 (1.6-7.1)], smoking [5.1 (1.3-9.3)], mode of delivery (CS) [3.2 (1.4-6.3)], age at first pregnancy (≥35 years) [7.4 (1.9-28.4)], blood group (AB) [3.3 (1.3-5.9)], gestational age (> 40 weeks) [3.1 (1.3-5.9)] and birth spacing (> 108 months) [4.8 (1.3-61.1)] were significant.
Conclusion: There is a significant burden of HDP (14.5%). PE and GH represented the highest followed by severe PE. A number of independent risk factors were identified and will help in screening women at higher risk of HDP.
Keywords
Hypertensive disorders in pregnancy, Behavioral risk factors, Hypertension, Pregnant women, Blood pressure, Preeclampsia, Gestational hypertension, Chronic hypertension
Hypertensive disorders in pregnancy articles Hypertensive disorders in pregnancy Research articles Hypertensive disorders in pregnancy review articles Hypertensive disorders in pregnancy PubMed articles Hypertensive disorders in pregnancy PubMed Central articles Hypertensive disorders in pregnancy 2023 articles Hypertensive disorders in pregnancy 2024 articles Hypertensive disorders in pregnancy Scopus articles Hypertensive disorders in pregnancy impact factor journals Hypertensive disorders in pregnancy Scopus journals Hypertensive disorders in pregnancy PubMed journals Hypertensive disorders in pregnancy medical journals Hypertensive disorders in pregnancy free journals Hypertensive disorders in pregnancy best journals Hypertensive disorders in pregnancy top journals Hypertensive disorders in pregnancy free medical journals Hypertensive disorders in pregnancy famous journals Hypertensive disorders in pregnancy Google Scholar indexed journals Behavioral risk factors articles Behavioral risk factors Research articles Behavioral risk factors review articles Behavioral risk factors PubMed articles Behavioral risk factors PubMed Central articles Behavioral risk factors 2023 articles Behavioral risk factors 2024 articles Behavioral risk factors Scopus articles Behavioral risk factors impact factor journals Behavioral risk factors Scopus journals Behavioral risk factors PubMed journals Behavioral risk factors medical journals Behavioral risk factors free journals Behavioral risk factors best journals Behavioral risk factors top journals Behavioral risk factors free medical journals Behavioral risk factors famous journals Behavioral risk factors Google Scholar indexed journals Hypertension articles Hypertension Research articles Hypertension review articles Hypertension PubMed articles Hypertension PubMed Central articles Hypertension 2023 articles Hypertension 2024 articles Hypertension Scopus articles Hypertension impact factor journals Hypertension Scopus journals Hypertension PubMed journals Hypertension medical journals Hypertension free journals Hypertension best journals Hypertension top journals Hypertension free medical journals Hypertension famous journals Hypertension Google Scholar indexed journals Pregnant women articles Pregnant women Research articles Pregnant women review articles Pregnant women PubMed articles Pregnant women PubMed Central articles Pregnant women 2023 articles Pregnant women 2024 articles Pregnant women Scopus articles Pregnant women impact factor journals Pregnant women Scopus journals Pregnant women PubMed journals Pregnant women medical journals Pregnant women free journals Pregnant women best journals Pregnant women top journals Pregnant women free medical journals Pregnant women famous journals Pregnant women Google Scholar indexed journals Blood pressure articles Blood pressure Research articles Blood pressure review articles Blood pressure PubMed articles Blood pressure PubMed Central articles Blood pressure 2023 articles Blood pressure 2024 articles Blood pressure Scopus articles Blood pressure impact factor journals Blood pressure Scopus journals Blood pressure PubMed journals Blood pressure medical journals Blood pressure free journals Blood pressure best journals Blood pressure top journals Blood pressure free medical journals Blood pressure famous journals Blood pressure Google Scholar indexed journals Preeclampsia articles Preeclampsia Research articles Preeclampsia review articles Preeclampsia PubMed articles Preeclampsia PubMed Central articles Preeclampsia 2023 articles Preeclampsia 2024 articles Preeclampsia Scopus articles Preeclampsia impact factor journals Preeclampsia Scopus journals Preeclampsia PubMed journals Preeclampsia medical journals Preeclampsia free journals Preeclampsia best journals Preeclampsia top journals Preeclampsia free medical journals Preeclampsia famous journals Preeclampsia Google Scholar indexed journals Gestational hypertension articles Gestational hypertension Research articles Gestational hypertension review articles Gestational hypertension PubMed articles Gestational hypertension PubMed Central articles Gestational hypertension 2023 articles Gestational hypertension 2024 articles Gestational hypertension Scopus articles Gestational hypertension impact factor journals Gestational hypertension Scopus journals Gestational hypertension PubMed journals Gestational hypertension medical journals Gestational hypertension free journals Gestational hypertension best journals Gestational hypertension top journals Gestational hypertension free medical journals Gestational hypertension famous journals Gestational hypertension Google Scholar indexed journals Chronic hypertension articles Chronic hypertension Research articles Chronic hypertension review articles Chronic hypertension PubMed articles Chronic hypertension PubMed Central articles Chronic hypertension 2023 articles Chronic hypertension 2024 articles Chronic hypertension Scopus articles Chronic hypertension impact factor journals Chronic hypertension Scopus journals Chronic hypertension PubMed journals Chronic hypertension medical journals Chronic hypertension free journals Chronic hypertension best journals Chronic hypertension top journals Chronic hypertension free medical journals Chronic hypertension famous journals Chronic hypertension Google Scholar indexed journals obstetricians articles obstetricians Research articles obstetricians review articles obstetricians PubMed articles obstetricians PubMed Central articles obstetricians 2023 articles obstetricians 2024 articles obstetricians Scopus articles obstetricians impact factor journals obstetricians Scopus journals obstetricians PubMed journals obstetricians medical journals obstetricians free journals obstetricians best journals obstetricians top journals obstetricians free medical journals obstetricians famous journals obstetricians Google Scholar indexed journals preeclampsia articles preeclampsia Research articles preeclampsia review articles preeclampsia PubMed articles preeclampsia PubMed Central articles preeclampsia 2023 articles preeclampsia 2024 articles preeclampsia Scopus articles preeclampsia impact factor journals preeclampsia Scopus journals preeclampsia PubMed journals preeclampsia medical journals preeclampsia free journals preeclampsia best journals preeclampsia top journals preeclampsia free medical journals preeclampsia famous journals preeclampsia Google Scholar indexed journals
Article Details
1. Background
According to the American college of obstetricians and gynecologist (ACOG), Hypertension (HTN) in pregnancy is defined as: Systolic blood pressure greater than or equal to 140 mmHg and/or diastolic blood pressure greater than or equal to 90 mmHg in two occasions at least 4-6 hours apart after fifth month of gestation for pregnancy induced hypertension or before 20 weeks of gestation for chronic hypertension. Hypertensive disorders of pregnancy (HDP) refers to categories of conditions characterized by elevated blood pressure and classified as chronic hypertension, preeclampsia superimposed on chronic hypertension, gestational hypertension, preeclampsia and eclampsia [1]. HDP is one of the most common complications in pregnancy forming a triad together with hemorrhage and infection. It affects about 10% of pregnancies [2-4] and contributes to significant maternal and perinatal mortality [5]. The World Health Organization (WHO) reported that 14.0% of global maternal deaths are attributed to HDP [6]. In Latin-American and Caribbean countries 25.7% of maternal deaths were due to HDP; in Asian and African countries, it contributed to 9.1% of maternal deaths and in fact about 16% in sub-Saharan African countries [5, 6]. In Cameroon, hypertension in Pregnancy (HIP) occurs in 7.7 – 8.2%. In the Far North Region, hypertension in pregnancy was the first cause of maternal death, representing 17.5% of the 63 maternal deaths recorded between 2003 and 2005 [7]. In the South West Region, the prevalence of PIH was 5.02% and Maternal Mortality was estimated at 1887/100,000 live birth [8]. In the centre region, Mboudou E. et al. [9] reported a prevalence of 8.2% with PE been the most frequent (77.9%), followed by GH (15.4%), superimposed PE (5.8%) and 0.96% been CH, with over 10.7% of the patients developing complications. Preterm delivery and low birth weight accounted for 30% of deaths due to HDP [10]. In the NWR, though Egbe et al., [11] showed that 14.5% of maternal deaths in Mezam Division were due to HDP, there are little or no published studies on the prevalence and risk factors of HDP. Thus, it will be imperative to contributes to the epidemiology of this disease in the NWR of Cameroon.
HDP is of global public health concern both in developed and developing countries. However, the risk that women
in developing countries die of HDP complications is approximately 300 times higher than that for women in developed countries. Women who developed pre-eclampsia were three times more likely to progress to eclampsia and if eclampsia develops, they are 14 times more likely to die of eclampsia [12]. HTN also raises the risk of heart attacks, cardiac failure, cerebrovascular accidents and renal failure in the mothers. The fetuses of hypertensive mothers are also at increased risks, such as: inappropriate placental oxygen transfer, Intra Uterine Growth Restriction (IUGR), premature delivery, placental abruption, stillbirth, and neonatal death [13]. Cognizant that the disease has no peculiar cause, several studies focusing on risk factors have been conducted in different parts of the globe and identified various risk factors for HDP. These factors can be grouped into modifiable and non-modifiable risk factors [14, 15]. The non-modifiable risk factors are traits or characteristics in the individual that cannot be changed or modified, hence they are out of our control and little or nothing can be done to change them. These factors include age, sex, race, gravidity, preexisting hypertension, family history, genetic composition [7, 16-18] etc. On the other hand, the modifiable risk factors are attributes, characteristics, exposures or life style patterns that can be adjusted or changed to avoid the development of the disease. The modifiable risk factors include; obesity, excessive salt intake, sedentary lifestyle/physical inactivity, high fat diet, tobacco use, alcohol consumption, caffeine [7, 17, 19] etc.
Smoking, alcohol intake, poor physical activity and insufficient fruit and vegetable intake are well-known modifiable behavioral risk factors of HDP [20, 21]. Addressing this risk factors is important not just for reducing adverse pregnancy outcomes such as low birth weight, fetal and infant mortalities, and potential congenital defects, but for decrease of HDP among mothers as well [20, 21]. In Nepal, a 2013 non-communicable disease risk factor survey (STEPS) revealed national prevalence of tobacco use to be 30.8%, alcohol intake of 17.4%, insufficient fruit and vegetable intake of 98.9%, and poor physical activity of 3.5% [22]. In addition, a higher percentage (15%) of pregnant women reported to consume alcohol during pregnancy [23]. However, studies of such have been limited in terms of describing the prevalence of behavioral risk factors and their predictors in Mezam division. Studies in Cameroon show that the prevalence of HDP ranges from 5.5-8.2% [7, 8, 17]. These HDP is one of the major causes of maternal and perinatal morbidities and mortalities. The Cameroon government have been planning to reduce maternal mortality due to pregnancy related complication like HTN, pre-partum and post-partum bleeding, but the incidence of HTN and its complication on mothers and new born is prevalent as indicted by different study conducted in different parts of the country [7, 17]. Though few studies have explored on the prevalence and associated determinants HDP in Cameroon, there are few/no study conducted yet in the current study area of interest. Therefore, this study was conducted to determine the prevalence and associated factors of HDP in Mezam division.
2. Materials and Methods
2.1 Study area/settings
The study was conducted in Mezam Division, NWR of Cameroon. The division is made up of five health districts of
which three (Bamenda, Tubah and Santa) were selected for the study. A total of ten facilities were purposefully selected for the study: Bamenda district (Regional hospital, Mulang HC, CMA Nkwen, Azire IHC, St. Blaise and St. Mary Hospital), Santa district (Santa DH and Akum IHC) and Tubah district (Tubah DH and CMA Bambili). The purposeful selection was due to the crisis situation in the NWR. The Division covers an area of 1745 km2, population size of 575, 312 and its elevation above sea level is 1332m (North West Regional Delegation of Public Health, 2014). Bamenda city is the administrative seat of Mezam Division and Regional Headquarters of the North West and the largest town in the North West Region. It is a cosmopolitan city which is the bedrock of Cameroon politics with a very strong traditional set up and extremely powerful Fons and Fondoms. The population though cosmopolitan has a greater majority of local ethnic groups, practice subsistence farming and can boast of a rich variety of food crops. The works of art of the local population is worth emulating as it projects the North West culture nationally and internationally. The colourfully designed traditional attire worn by both men and women depicts a culture which has stood the test of time. The town is endowed with touristic potentials owing to its landscape characterized by waterfalls, craters and traditional palaces. Subsistent farming, long distant trekking and petit trading characterise women of this region, keeping some of the women very active. However, the crisis in this region has limited these women from their activeness and equally contributed to little/poor ANC follow-up, as a result might contribute to development of HDP. Thus, our study is concern with the prevalence and risk factor of HDP in the Mezam division, NWR of Cameroon.
2.2 Study period
Data was collected for a period of four months i.e. March-June, 2018.
2.3 Study design and population
Health facility based cross-sectional study design with quantitative data collection method was used. All women who attending ANC services in the selected facilities were considered as source population whereas all sampled women were considered as study population.
2.4 Inclusion and exclusion criteria
Living and attending ANC within the study area (Mezam Division), women of reproductive age (12-49 yrs.) and be of gestational age ≥36 weeks were included in the study. Pregnant women with hearing problems and those with severe mental retardation were excluded from the study.
2.5 Sample size and sampling technique
The sample size for the cross-sectional study was calculated by using a single population proportion sample size calculation formula by considering the prevalence (P) of 8.2% [9]. Because the prevalence is < 10%, the margin of error (precision) will be 8.2%/5= 1.64% [24] at 95% confidence interval (CI). By considering 10% none response rate, the final sample size will be calculated as follows;
n= Z P (1-P)/d2 (25)
n = Number of participants for the study,
Z= 1.96 at 95% confidence interval.
P= 8.2% = 0.082,
d = 8.2/4 (because sample size is <10%) = 2.05%,
q= 1- p = 1-0.082 = 0.918
n= (1.96)2×0.082× (1-0.082)/ (0.0164)2 = 1,075.2 ≈ 1,076. Considering 10% of 1,075=107.6
Therefore 1,076 + 107.6 = 1184. However, the final sample size reached was 1210.
The total sample size was proportionally allocated to the ten facilities based on their source population. The source population of each hospital was taken from the Regional Delegation of Health, NWR. Afterwards, the study participants were consecutively selected from each hospital until the required sample size was reached.
2.6 Data collection
The questions were grouped/arranged according to particular objectives that the study aimed to address. Six data collectors’ midwives in qualification (fluent in pidgin and English) were recruited to collect data for the study. Preliminary questionnaires were checked, pre-tested in two facilities and necessary modifications made. The questionnaire consisted of three parts: i) baseline characteristics of respondents; ii) reproductive history of respondent and iii) behavioral risk factors of hypertension (tobacco use, alcohol consumption, fruit and vegetable consumption, stress, salt intake, overweight/obesity and physical activity). The participants were asked whether/not they smoke, drink, consumed much salts, quantity/frequency of fruits and vegetable intake (show cards regarding fruits and vegetables intake during pregnancy was used), participants were asked whether/not they were stressed during pregnancy, height and weight were captured from participants ANC cards. The World Health Organization (WHO) global recommendations on PA for health guideline to measure PA level among pregnant women in this study [26]. Activities considered during PA were; farming, walking, jogging, home activities, time spent in walking/sitting at job side. The Compendium of PA was used to calculate total MET/min/week spent (Compendium of PA, 2005). Data was collected by face-to-face interviews of respondents for about 25 minutes. Before data collection, the interviewers were trained for two days on the tools to be used, purpose of the study and how to approach respondents and obtain consent.
2.7 Data management and analysis
Two data supervisors were recruited to monitor data collection daily. The questionnaires were checked for completeness and correctness. The collected data was entered into Excel, cross-checked for accuracy and validity. The computer in which the data was entered was pass worded. Unique Identification Codes (UIC) was used to ensure participants confidentiality. Data was analysed using Statistical Package for Social Sciences (SPSS) version 21 (IBM - SPSS, Inc, Chicago, IL, USA) and Epi INFO version 7 (CDC). Frequencies, proportions and percentages were used for descriptive analysis. Multivariate logistic regression was used to determine the adjusted associations between the demographic variables and the behavioural risk factors of hypertension. It was determined a priori that explanatory variables with p-value ≤ 0.2 were considered for multivariate analysis. Variables with p-value < 0.5 were considered significant for the behavioural risk factors.
2.8 Ethical Clearance
Ethical clearance for the study was obtained from the Faculty of Health Sciences institutional review board
FHSIRB), university of Buea. In addition, administrative authorizations were obtained from the Regional Delegation of Public Health (RDPH), North West Region (NWR) and the District Health Officers (DHOs) of the Bamenda Health District (BHD), Santa Health District (SHD) and Tubah Health District (THD) and from Directors of all the health facilities included in the study. Potential respondents gave verbal and written/signed consent after they had been given an explicit explanation of the study and an opportunity to ask and respond to any questions. Ascent was also sort from participants less than 18 years old. The questionnaires were anonymous and answers were kept confidential.
3. Results
3.1 Socio-demographic characteristics of participants
Table 1 presents socio-demographic characteristics of 1210 participants who took part in the study. The mean and standard deviation (SD) age of participants was 26.9 (5.41). From the table, over a third of the pregnant women 455 (37.6%) & 390 (32.3%) were between ages ≤24 years & 25-29 years old respectively. The mean/standard deviation (SD) age of participants was 26.9 (5.4) respectively. A fifth 252 (21.6%) of the respondents were single while close to four fifth were 949 (78.4%) were married/cohabiting. Majority of the participants 422 (34.9%) had attended secondary education while an almost equal proportion, 292 (24.1%) and 286 (23.6%) had attended High School and Tertiary education respectively. As concerns occupation, students made up a quarter of the population 321 (26.5%), 210 (17.4%) were peasant/housewives and just 274 (22.5%) were employed or doing large scale business. Residence of Bamenda District were the most represented (69.2%), followed by Santa District (17.0%). Regarding religion, close to three quarter 878 (72.6%) were Catholic/Protestant and almost a quarter 263 (21.7%) Pentecostal (Table 1).
|
Variables |
Respondent |
Percentages (%) |
|
Age of participants (years) |
||
|
Mean (SD) |
26.9 (5.4) |
|
|
≤24 25-29 30-34 ≥35 |
455 390 240 125 |
37.6 32.3 19.8 10.3 |
|
Marital Status |
||
|
Married/cohabiting Single/separated |
949 261 |
78.4 21.6 |
|
Educational Status |
||
|
None/Primary Secondary High school Tertiary |
210 422 292 286 |
17.4 34.9 24.1 23.6 |
|
Occupation |
||
|
Peasant/Housewife Student Petit Trader Large scale business/Employed |
210 321 407 272 |
17.4 26.5 33.6 22.5 |
|
Area/District of Residence |
||
|
Bamenda district Santa District Tubah District |
873 206 131 |
69.2 17.0 13.8 |
|
Religion |
||
|
Catholic/Protestants Pentecostal Muslims |
878 263 69 |
72.6 21.7 5.7 |
Table 1: Socio-demographic characteristics of pregnant women (n=1210).
3.2 Prevalence of hypertension in pregnancy
The prevalence of hypertension among the study participants (pregnant women) was 14.5% (176/1210) (Confidence Interval (95%CI): 12.6% -16.7%) (Figure 1).
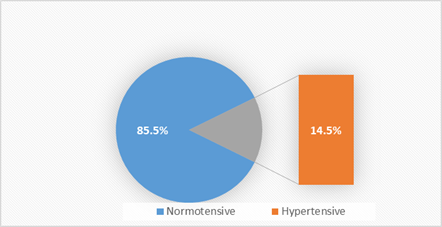
Figure 1: Prevalence of hypertension in Pregnancy.
3.3 Prevalence of hypertension by Systolic, Diastolic and/both
Of the 14.5% who suffered hypertension in pregnancy, 6.4% suffered high blood pressure by elevated systolic blood pressure only, 4.6% suffered HBP by elevated DBP only while 3.4% had both elevated systolic and diastolic blood pressure (Figure 2).
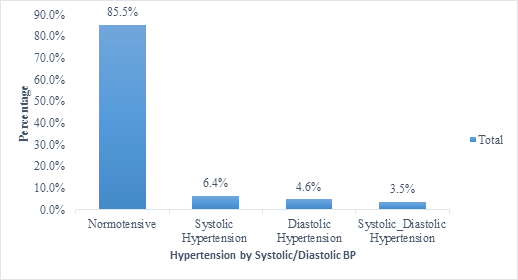
Figure 2: Prevalence of hypertension by Systolic, Diastolic and/both.
3.4 Hypertensive disorders in pregnancy (types)
Of the pregnant women who were hypertensive, 3.4% suffered chronic hypertension (CH), 5.7% suffered preeclampsia superimposed on chronic hypertension (PSCH), 31.8% suffered gestational hypertension (GH), 48.3% suffered preeclampsia (PE) and 10.8% suffered severe preeclampsia (SPE) (Figure 5).
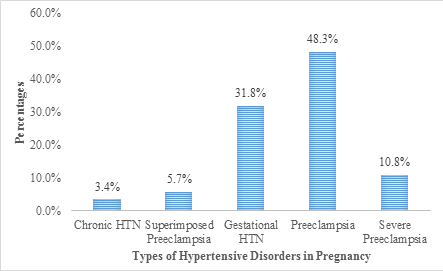
Figure 3: Hypertensive Disorders in Pregnancy (types).
3.5 Prevalence of hypertension by Age, Health District and Trimester of visit
The prevalence of hypertension by age group 14-24, 25-29, 30-34 and ≥ 35 years was 13.4%, 10.3%, 20.4% and 20.8% respectively. The Pearson Chi-Square for age was X2 (3) = 16.84, p = .001. This implies that there is a statistically significant association between age of participants and hypertension in pregnancy. The strength of association was measured by Phi and Cramer’s test which equally proved strong significance. The prevalence of hypertension by Health District was 14.2%, 17.5% and 12.2% respectively for Bamenda, Santa and Tubah. The Pearson Chi-Square for health District was X2 (2) = 2.07, p = .354. This implies that there is no statistically significant association between Health District and the prevalence of hypertension in pregnancy. The prevalence of hypertension by Trimester of visit was 16.5%, 14.3% and 14.5% respectively for first, second and third trimester. The Pearson Chi-Square by trimester of visit was X2 (2) = 0.402, p = .838. This implies that there is no statistically significant association between trimester of visit and the prevalence of hypertension in pregnancy (Table 2).
|
Variables |
Proportion/% Non-Hypertensive |
Proportion/% Hypertensive |
χ2 value (df) |
P-value |
|
Age of pregnant women (years) |
||||
|
≤ 24 25-29 30-34 ≥ 35 |
394/455 (79.6) 350/390 (89.7) 191/240 (79.6) 99/125 (79.2) |
61/455 (13.4) 40/390 (10.3) 49/240 (20.4) 26/125 (20.8) |
16.84 (3) |
.001* |
|
Health Districts |
||||
|
Bamenda Santa Tubah |
749/873 (85.8) 170/206 (82.5) 115/132 (87.8) |
124/873 (14.2) 36/206 (17.5) 16/131 (12.2) |
2.07 (2) |
.354 |
|
Trimester of visit |
||||
|
First trimester Second trimester Third trimester |
96/115 (83.5) 796/929 (85.7) 142/166 (85.5) |
19/115 (16.5) 133/929 (14.3) 24/166 (14.5) |
0.402 (2) |
.818 |
*significance at p< 0.05
Table 2: Prevalence of hypertension by Age, Health District and Trimester of visit.
3.6 Risk factors of hypertensive disorders in pregnancy
Table 3 reveals the adjusted logistic regression analysis of chronic HTN, Gestational HTN and PE in Mezam Division. For chronic HTN, after bivariate analysis, the following factors with P-values ≤0.2 were considered for multivariate analysis; family history of HDP, daily fruits/vegetable intake, stress, being overweight/obese, HTN in previous pregnancy and method of birth control. In the multivariate regression analysis, after controlling for potential confounders, four factors were found to be independently associated with chronic HTN; women with family history of HDP were 3.70 (95%CI: 1.7-11.6) times more likely to suffer Chronic HTN compared to those with no family history of HDP. Women who were stressed-up were 2.80 (95%CI: 1.1-10.9) times more likely to suffer Chronic HTN compered to their counterparts. Overweight/obese women had 2.6 (95%CI: 1.3-6.7) odds of developing chronic HTN compared to their counterparts.
On the other hand, women with history of chronic HTN were 0.4 (95%CI: 0.1-0.9) protective from developing Chronic HTN. For Gestational HTN, after bivariate analysis, fourteen factors with P-values ≤0.2 were considered for multivariate analysis; age, marital status, area/district of residence, religion, women with family history of HDP, family history of GD, smoking, alcohol consumption, gravidity, parity, number of ANCs attended, trimesters of visit, HTN in previous pregnancy and blood group. At the multivariate level, after controlling for potential confounders, seven factors were found to be independently associated with Gestational HTN. Women of Age 25-29 were 0.3 (95%CI: 0.1-0.9) less likely to suffer Gestational HTN compared to those of other age groups. Single/separated women were more at risk of GH (2.7 (95%CI: 1.5-4.7)). Pregnant women with family history of HDP were 4.40 (95%CI: 1.5-13.1) times at risk of GH. Consumers of alcohol were 2.8 (95%CI: 1.1-5.4) times at risk of Gestational HTN compared to non-drinkers. Women with a history of HDP had a 4.9 (95%CI: 1.5-10.2) increase odds of developing Gestational HTN compared to their counterparts. For number of ANCs attended, women with 3-5 and ≥ 6 ANCs had a 0.5 (95%CI: 0.2-0.9) and 0.4 (95%CI: 0.2-0.9) reduced odds respectively of developing Gestational HTN. Similarly, women with blood group B had a 0.1 (95%CI: 0.0-0.6) reduced odds of developing Gestational HTN compared to women of other blood groups.
Considering PE, after bivariate analysis, twenty-one factors with P-values ≤0.2 were considered for multivariate analysis; age, marital status, occupation, family history of GD, smoking, alcohol consumption, gravidity, parity, abortion, number of ANCs attended, multiple pregnancies/twin, HTN in previous pregnancy, previous mode of delivery, LBW, age difference between pregnancies, UTI, gestational age at last ANC and blood group. At the multivariate level, after controlling for potential confounders, seven factors were found to be independently associated with PE. Overweight/obese women had 2.7 (95%CI: 1.4-7.1) odds of developing PE compared to their counterparts. On the other hand, women with history of HDP were 3.5 (95%CI: 1.6-7.1) more likely to develop PE compared to those free of HDP before. Pregnant women who smoke had a 5.1 (95%CI: 1.3-9.3) odds of developing PE compared to non-smokers. Women who delivered by induced labour had a 0.3 (95%CI: 0.1-0.9) reduced odds of developing PE while those that delivered by CS had a 3.2 (95%CI: 1.4-6.3) increase odds of developing PE compared to their counterparts. For age at first pregnancies, women who were pregnant at ages 25-29 had a reduced odd 0.3(95%CI: 0.1-0.8) of developing PE compared to older pregnant women of ≥35 years of age with a 7.4 (95%CI: 1.9-28.4) increase odds of developing PE. Similarly, for gestational age at delivery, women with gestational age > 40 weeks had a 3.1 (95%C: 1.3-5.9) increase odds of developing PE compared to those of younger group. For women with blood group AB, there was a 3.3 (95%CI: 1.3-5.9) increase odds of developing PE compared to women of other blood groups. Lastly, for gap between pregnancies, women with gap > 108 months had a 4.8 (95%CI: 1.3-61.1) increase odds of developing PE compared to their counterparts (Table 3).
|
Variables |
Multivariate Logistic Regression |
||
|
Chronic HTN |
Gestational HTN |
Preeclampsia |
|
|
AOR (95% CI) |
AOR (95% CI) |
AOR (95% CI) |
|
|
Age (years) |
|||
|
≤24 25-29 30-34 ≥35 |
1 0.4(0.1-1.6) 0.3(0.2-1.6) 0.8(0.2-3.5) |
1 0.3(0.1-0.9)* 0.6(0.3-1.3) 0.7(0.3-1.8) |
1 1.1(0.4-2.9) 2.2(0.7-4.6) 3.6(0.9-7.9) |
|
Marital Status |
|||
|
Married Single/Separated |
1 0.5(0.1-2.3) |
1 2.7(1.5-4.7)* |
1 2.4(0.8-4.6) |
|
Educational Status |
|||
|
None/Primary Secondary High school Tertiary |
1 1.2(0.2-6.4) 1.8(0.3-9.4) 1.4(0.3-8.1) |
1 1.2(0.5-2.5) 0.9(0.4-2.0) 0.8(0.3-1.9) |
1 1.6(0.9-3.1) 0.9(0.4-2.0) 1.0(0.4-2.3) |
|
Family history of HDP |
|||
|
No Yes |
1 3.7(1.7-11.6)* |
1 4.4(1.5-13.1)* |
1 0.9(0.4-2.1) |
|
Smoking? |
|||
|
No Yes |
1 0.2(0.01-0.5) |
1 2.3(0.3-18.6) |
1 5.1(1.3-9.3)* |
|
Alcohol? |
|||
|
No Yes |
1 0.8(0.2-2.9) |
1 2.8(1.1-5.4)* |
1 2.1(0.3-4.9) |
|
Physical Activity (MET/Min/Week) |
|||
|
≥600 MET/Min/Week <600 MET/Min/Week |
1 1.0(0.4-2.8) |
1 1.2(0.7-2.1) |
1 1.2(0.8-1.8) |
|
Daily Fruits & Vegetable intake? |
|||
|
≥ 5 servings < 5 servings |
1 0.4(0.1-1.5) |
1 1.1(0.5-2.2) |
1 1.2(0.7-2.0) |
|
Stress? |
|||
|
No Yes |
2.8(1.1-10.9)* |
1.3(0.8-2.3) |
1.1(0.6-1.9) |
|
BMI of Participants (kg/m2) |
|||
|
<25 ≥25 |
2.6(1.3-6.7)* |
0.7(0.4-1.3) |
2.7(1.4-7.1)* |
|
Age at first pregnancy |
|||
|
≤24 25-29 30-34 |
1 1.6(0.3-2.6) 2.3(0.6-2.8) |
1.6(0.2-12.3) 0.8(0.1-8.8) |
0.3(0.1-0.8) 0.9(0.1-9.9) |
|
≥35 |
3.4(0.8-6.9) |
2.2(0.1-36.3) |
7.4(1.9-28.4)* |
|
HTN in previous pregnancy? |
|||
|
No Yes |
1 0.4(0.1-0.9)* |
1 4.9(1.5-10.2)* |
1 3.5(1.6-7.1)* |
|
Previous Mode of Delivery |
|||
|
Normal Induced Cesarean section |
1 0.6(0.1-4.5) 1.6(0.3-7.6) |
1 1.2(0.5-3.0) 0.3(0.1-2.1) |
1 0.3(0.1-0.9)* 3.2(1.4-6.3)* |
|
Age Difference between birth (Months) |
|||
|
≤18 18-60 61-108 >108 |
1 1.0(0.3-3.0) 1.5(0.3-7.6) 0.3(0.01-0.8) |
1 1.6(0.2-12.3) 0.8(0.1-8.8) 2.2(0.1-36.4) |
1 1.4(0.2-12.3) 2.6(0.3-24.2) 4.8(1.3-61.1)* |
|
Blood Group |
|||
|
A B AB O |
1 0.5(0.1-2.7) 0.9(0.1-8.9) 0.9(0.3-2.8) |
1 0.2(0.0-0.6)* 1.9(0.5-8.4) 0.6(0.3-1.5) |
1 2.3(0.8-2.9) 3.0(1.3-5.9)* 1.6(0.7-3.7) |
|
Trimester of Visit |
|||
|
First Second Third |
1 0.5(0.1-1.9) 0.2(0.03-2.1) |
1 1.8(0.6-5.9) 2.4(0.6-8.9) |
1 0.7(0.4-1.3) 0.7(0.3-1.6) |
|
Gestational age at last ANC |
|||
|
< 37 weeks 37-40weeks >40 weeks |
1 1.1(0.1-2.2) 1.0(0.01-1.9) |
1 0.5(0.2-1.2) 0.6(0.1-2.1) |
1 0.8(0.4-4.2) 3.1(0.2-12.1) |
|
Number of ANCs Attended |
|||
|
1-2 3-5 6-8 |
1 0.4(0.02-5.5) 0.3(0.01-4.1) |
1 0.5(0.2-0.9)* 0.4(0.2-0.9)* |
1 0.7(0.7-2.1) 1.3(0.4-4.0) |
Table 3: Risk factors of hypertensive disorders in pregnancy.
4. Discussion
Hypertensive disorders in pregnancy (HDP) remain a major public health problem worldwide, with varying prevalence from country to country. In this study the prevalence of HDP was 14.5% which is higher than the 5.02% reported by Halle-Ekane et al., [8] in Fako Division and two other studies carried out in Yaoundé Cameroon, with prevalence of 7.7% and 8.2% respectively [27]. It is equally higher than the 5.5% reported in the Far North Region of Cameroon [7]. A study in a Nigerian teaching hospital reported an incidence of 6.2% [28] and another in Ethiopia reported a prevalence of 8.48% [29] which are all lower than that of the current study. The variations in this prevalence may be due to the difference in health facility types, settings, geographical locations, study designs, years and periods during which the study was carried out. However, it was consistent to the 17% reported by Singh et al. [30], 17.2% that had been reported in Finland [13] and the 12 - 22% of all pregnancies reported by the American College of Obstetricians and Gynecologist (depending on the population and the definitions used) (ACOG Practice Bulletin No. 33; 2002). On the other hand, the prevalence was lower than the 31.1% observed in the Moliwe Health Area, South West Region, Cameroon [31] and the 31.0% reported in Douala, Cameroon [32]. It was also by far lower than the 32.9% reported by Amoah [33] in semi-urban Ghana. The high prevalence of HDP determined in this study might be attributed to the settings and current crises in North West and South West Regions where most of the high-risk obstetric cases and emergencies are not referred from primary and secondary health facilities. It is most probable that majority of the uncomplicated cases of pregnancies are managed at level of the primary whilst the complicated ones including HDP are referred to the secondary center for specialized multidisciplinary care. This was a hospital-based study carried out in a purely African setting with diverse socio-economic features and this could partly account for the higher prevalence of HDP compared to a population-based study. This study also determined the frequencies of the various types of HDP in Mezam. The proportion of PE among all the women with HDP was 48.3% which was the highest, followed by GH (31.8%), severe PE (10.8%), superimposed PE on chronic HTN (5.7%) and CH (3.4%). The results of this study ties with a recent study from Nigeria by Olusanya and Solanke who reported the frequency of 4.6% for CH, 55.6% for GH and 39.8% for PE [28]. The findings also concur with a study in Ghana with reported proportion of GH among all the women to be (50.0%), followed by PE (38.0%), chronic HTN (6.3%) and superimposed PE on CH (5.7%) [34]. The prevalence of HTN by age group 14-24, 25-29, 30-34 and ≥ 35 years was 13.4%, 10.3%, 20.4% and 20.8% respectively. According to the Pearson Chi-Square, there was a significant association between age and HDP (χ2 (3) = 16.84, p = .001). Pregnant women of ages 30-34 and ≥ 35 recorded the greatest prevalence. Some studies have reported association between age and HDP especially in elderly women above the age of 35 years, while others have shown an association of PE with younger age groups. Advancing maternal age as well as young maternal age is a risk factor for HDP [35, 36].
4.1 Independent risk factors of HDP
4.1.1 Chronic hypertension: After a multivariate analysis, the following factors were independently associated with CH; women with family history of HDP (OR: 3.70, 95%CI: 1.7-11.6), Women who were stressed (OR: 2.80, 95%CI: 1.1-10.9) and women with BMI ≥25Kg/m2 (OR: 2.6, 95%CI: 1.3-6.7) odds of developing CH compared to their counterparts. CH was more common in women whose mothers had experienced HDP [37, 38] and in pregnancies fathered by sons of women who had PE [39]; from these findings, it seems that both maternal and fetal genes play a role in this syndrome. Therefore, pregnant women with a family history of HTN should be monitored carefully both prenatally and in the postpartum period [38, 40]. In the study by [41], the incidence of CH was significantly higher in women with greater stress score compared to women with no stress during pregnancy. The increased incidences of CH in women with mental stress could be explained based on the facts that psychosocial strain results to vasoconstriction and increased uterine artery resistance, which results in development of HTN [42]. On the other hand, women with history of CH were 0.4 (95%CI: 0.1-0.9) protective from developing CH. The findings contrast the results of Tesfaye et al. [43], who showed that women with family history of HTN had approximately ten-fold odds of developing CH.
4.1.2 Gestational hypertension: In our findings, women of ages 25-29 years were (OR: 0.3, 95%CI: 0.1-0.9) less likely to develop GH. Age group-specific prevalence had shown a steep decline after maternal age of 19 years followed by a general increase with the highest rate occurring after maternal age of 35 years in this study. Extreme ages of reproductive years are well known risk factors for GH with high incidence rates in teenagers [44]. Many authors have identified young age as a risk factor for HDP. Adeyinka et al. [45] found the prevalence of HDP among adolescents to be 20% in comparison to only 3.33% among the controls. In another study, a 2.9% vs. 0.6% GH was reported in teenagers compared to women aged 25-34 years [46]. Tebeu et al., [7], reported that the number of early teenagers among patients with HTN was 16.4% compared to 6.8% among the controls. Marital status had been a significant risk factor for HDP. In our study, unmarried (single/separated) women were (OR: 2.7, 95%CI: 1.5-4.7) times more likely to develop GH. Similar results were obtained by Tessema et al., [47] in which women currently unmarried were about three times more likely to develop PE than those who were married. In another study in USA [48], singleton was more likely to develop GH. This could be explained by the possibility of low preconception period seminal fluid exposure between the women and their partners. This seminal familiarity would obtain from frequent insemination by the same partner for long period of time and hence could lead to the development of HDP [49]. On the other hand, it might also be explained by stress that arisen from delivering a child without father brought less social acceptability and economical support.
In the current study, women with previous history of HTN had (OR: 4.9, 95%CI: 1.5-10.2) had an increased risk of developing GH. In one study by Mehta et al., [22], the prevalence of HTN in pregnancy was found to be significantly higher in women with HTN in previous pregnancy (40.5%) compared to their counterpart (5.5%). Logistic regression by Mehta et al., [22] showed that GH was ≈11times more likely to occur in women with history of HTN in previous pregnancy. Similarly, Nisaret al., (2010) and Tebeuet al., (2011) found significant association between history of HTN in previous pregnancy and no HTN. There are consistent findings of a positive association between family histories of HDP risk among pregnant women [36]. The current study revealed that women with family history of GH had (OR: 4.40, 95%CI: 1.5-13.1) increase risk of developing GH. Tessema et al., [47] showed that women with family history of HTN had about seven times greater odds of developing GH compared those with no history of HTN. The study is also in line with studies conducted in Brazil [40], Sudan [50], Pakistan [51], and Uganda [52]. Family history of HTN is a proxy measure for hereditary factors as well as common environmental or behavioral exposures that may underlie HDP risk. Family history of HTN is an important and easy to acquire clinical risk marker for HDP compared to the biochemical markers. Alcohol intake has long been a contributing factor to overweight due to the number of calories it contains [53]. In the current study pregnant women who were consumers of alcohol were (OR: 2.8, 95%CI: 1.1-5.4) times likely to develop GH compared to non-drinkers. A study by Yamada et al., [54] confirmed that there was rise in systolic and diastolic blood pressure in persons consuming 24 to 30 grams of alcohol per day compared to non-drinkers.
In this study, pregnant women with ‘B’ blood group were (OR: 0.1, 95%CI: 0.0-0.6) less likely to suffer GH. A significant association was found between the ABO blood group and HDP. Findings from Mishra & Pradhan, [55], support the hypothesis that genetic factors related to the distribution of some blood groups may play a role in the development of HDP. According to Mishra & Pradhan, [55], women with group-A blood types have significantly higher risk (63%-81%) of HDP as compared to ‘B’ type individuals. Number of ANCs attended is a significant risk factor of GH. Mothers who attended <3 ANCs are more likely to suffer GH. In a study by Zenebe et al., [56], twenty six percent of 153 mothers with the disease had never had ANC visit during the index pregnancy. The results are in-line with results of the current study which shows that women with 3-5 (OR: 0.5, 95%CI: 0.2-0.9) and ≥ 6 ANCs (OR: 0.4, 95%CI: 0.2-0.9) had lesser risk of GH. However, other studies have proven a contrary. A study by Zenebe et al., showed that mothers with more ANCs were found to be affected more with severe forms of HDP compared to those with fewer ANCs though results were not statistically significant (p= 0.066) [56].
4.2 Preeclampsia and its risk factors
In the current study, overweight/obese women had 2.7 (95%CI: 1.4-7.1) increase risk of developing PE compared to their counterparts. With regard to the influence of BMI, O’Brien et al. [57] reported that the risk of PE increases with increase in BMI. The reason for this is probably the increased shear stress caused by hyper dynamic circulation which is associated with obesity [58]. Dalmáz et al. [40] also mentioned that being overweight (obesity and pre-obesity) correlated with an increased risk of developing PE. In addition, a study conducted by Gaio et al. [59] found obesity to be a risk factor for PE/eclampsia and chronic hypertension, and Assis et al. [39]; Suleiman, [38] demonstrated that obesity increases the risk of HDP. The worldwide increase in the frequency of obesity is likely to also increase the frequency of PE. In this study, women with previous history of HDP were 3.5 (95%CI: 1.6-7.1) more likely to develop PE compared to women with no history of HDP. In one study by Mehta et al., [22], the prevalence of HTN in pregnancy was found to be significantly higher in women with history of HTN in previous pregnancy (40.5%) compared to those with no history of HTN in previous pregnancy (5.5%). Similarly, Nisaret al., [60] and Tebeuet al., [7] found significant association between history of HTN during previous pregnancy and HTN in current pregnancy. Looking at previous mode of delivery, women who delivered by induced labour had a 0.3 (95%CI: 0.1-0.9) reduced odds of developing PE while those that delivered by caesarean section (CS) had a 3.2 (95%CI: 1.4-6.3) increase odds of developing PE. It is important to emphasize that previous CS is a risk factor for hypertension. The primary CS rate among women who reported HTN during pregnancy in one study was 33%, a significantly higher rate than among women who were not hypertensive (19%) [61]. PE was significantly higher in women with previous history of CS (17.6 vs 6.5%) than women with no CS [62]. Prior CS was found to be associated with an increased risk of having PE in the second pregnancy (OR, 1.26; 95% CI, 1.13–1.41) [62]. In a study to evaluate pregnancy-related HTN in multigravidas with previous CS, the occurrence of HTN was significantly higher (OR: 2.9, 16% vs. 6.7%, p=0.01) in prior cesarean multigravidas [63].
In this study, age at first pregnancy was found to be a significant factor for PE. Women who were pregnant at ages 25-29 had a decrease risk 0.3 (95%CI: 0.1-0.8) of developing PE compared to women ≥35 years who had a 7.4 (95%CI: 1.9-28.4) increase risk. Extreme ages of reproductive years are well known risk factors for HDP with high incidence rates in teenagers [44]. Many authors have identified young age as a risk factor for HTN during pregnancy. Adeyinka et al. [45] found the prevalence of HDP among adolescents to be 20% in comparison to only 3.33% among the controls. In another study, a 2.9% vs. 0.6% GH was reported in teenagers compared to women aged 25-34 years [46]. Tebeu et al., [7], reported that the number of early teenagers among patients with HTN was 16.4% compared to 6.8% among the controls. In the current study, Pregnant women who smoke had a 5.1 (95%CI: 1.3-9.3) odds of developing PE compared to non-smokers. Cigarette smoking is known to have adverse effects on all organ systems. However, a systematic review of 48 epidemiological studies reported that smoking during pregnancy approximately halves the risk of pre-eclampsia [64]. The pathophysiology of this relationship is not well established. However, it is proposed that smoking might have effects on angiogenic factors (factors that increase growth of new blood vessels from existing ones), endothelial function and the immune system, which may contribute to the lowered risk of pre-eclampsia. For gestational age at delivery, women with gestational age > 40 weeks had a 3.1 (95%CI: 1.3-5.9) increase risk of developing PE. Tesfaye & Tilahun, [65], identified gestational age as a predictor of PE, and showed that women with gestational age 37-39 weeks were less likely to develop PE by 9.6% compared to women with gestational age < 37weeks [(AOR=0.096 at 95% CI (0.04-0.23) [65]. Shikha Saxena et al., [66], reported that, overall, majority of the HDP subjects belonged to more than 36 weeks of gestational age (54.29%) followed by the gestational age of 28 - 36 weeks (38.57%). Thus, it was noted that there was a higher incidence of HDP in women with higher gestational age.
Women with blood group ‘AB’, had a 3.3 (95%CI: 1.3-5.9) increase odds of developing PE compared to women of other blood groups. Findings from Mishra & Pradhan [55], support the hypothesis that genetic factors related to the distribution of some blood groups may play a role in the development of HDP. Manjunatha S et al. [67] showed that women with blood group ‘AB’ had the highest risk for PE compared to other blood groups. In a study on British women with ‘AB’ blood type researchers found 2.7-fold risk of PE compared with ‘O’ type individual [68]. Similarly, other studies found an increased risk of PE by 2.1 to 3.1folds in Italian and Finnish pregnant women with AB blood type compared with O type women [69]. Considering pregnancy gap (age difference between pregnancy), women with pregnancy gap > 108 months had a 4.8 (95%CI: 1.3-61.1) increase risk of developing PE. From the review of literature, it is well recognised that either a too short (<2 years) or too long (>10 years) birth interval increases a woman's risk for developing PE (Magee et al., 2014). It has been suggested that a prolonged interval between pregnancies is a major risk factor of PE [70, 71]. In particular, the risk of PE in the second pregnancy seemed to surge at approximately 5 years after the first delivery [71], meanwhile the risk after 10 years was similar to that among nulliparous women, suggesting that the benefit of higher parity in terms of PE risk is only transient [70]. The results of this research work are generally applicable to clinical practice in our sub-region and more specifically Cameroon as this represents baseline prevalence study in the region. Based on results of the study, we recommend a larger cross-sectional/longitudinal study with adequate power to better understand the relative prevalence and incidence of HDP in Cameroon and why not Sub-Saharan Africa at large. In the nearest future, we plan to scale up this study to involve the community to determine the population-based prevalence to provide a true reflection of the burden of HDP in the country.
5. Conclusion
There is a significant burden of HDP in Mezam Division as evidenced by a high prevalence of 14.5%. Of which 3.4% was CH, 31.8% GH, 48.3% PE, 5.7% PE superimpose on CH and 10.8% severe PE. Family history of HDP, stress, overweight/obese, single/separated, alcohol, maternal history of HDP, smoking, mode of delivery (CS), age at first pregnancy (≥35 years), blood group (AB), gestational age (> 40 weeks), and birth spacing (> 108 months) were independent risk factors of HDP. Knowledge of risk factors of HDP necessitates strong behavioural and none-behavioral risk factor education in our community/HF during pregnancy and even the time preceding pregnancy to create greater awareness of the danger signs of HDP. Prevention strategies must include campaigns and education from the medical and paramedical staff. The confirmation of these findings should help towards the development of the national strategies of HDP prevention.
Acknowledgements
The authors acknowledge the contribution of the pregnant women participated in the study. The authors also acknowledge the assistance of the Regional Delegate of Public health for North West Region, the District Health Officers and Directors of all facilities that were part of the study.
Conflict of Interest
We declare no conflict of interest.
References
- Cunnigham FG, Leveno K, Bloom Sea. Williams Obstetrics. Edition r, editor. Medical Publishing Division (2010).
- Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/Eclampsia. Semin Perinatol 36 (2012): 56-9.
- Organization WH. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. World Health Organization, Dept. of Reproductive Health and Research, Dept. of Maternal, Newborn, Child and Adolescent Health, Dept. of Nutrition for Health and Development (2011).
- WHO U, UNFPA, The World Bank. Trends in maternal mortality 1990-2008: Estimates developed by WHO, UNICEF, UNFPA and the World Bank. 2010. World Health Organization (2012).
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 367 (2006): 1066-1074.
- Say L, Chou D, Gemmill A, Moller AB, Daniels J, Temmerman Me. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2 (2014): 323-333.
- Tebeu PM, Foumane P, Fosso G, Bivaga PT, Fomulu JN. Risk Factors for Hypertensive Disorders in Pregnancy: A Report from the Maroua Regional Hospital, Cameroon. J Reprod Infertil 12 (2011): 227-234.
- Halle-Ekane GE, Che M, Atashili J, Bechem NN, Nsagha DS, Thomas ETO, et al. Severe Pregnancy Induced Hypertension, a Dreaded Complication in Semi-Urban Area in Fako Division, Cameroon? A Case – Control Study. British Journal of Medicine & Medical Research 12 (2016): 1-9.
- Mboudou ET, Foumane P, Belley Priso E, Dohbit J, ZE MJ, Nkengafac WM, et al. HYPERTENSION AU COURS DE LA GROSSESSE: Aspects cliniques et épidémiologiques a l’Hôpital Gynéco-Obstétrique et Pédiatrique de Yaounde, Cameroun. Clinics in Mother and Child Health 6 (2009): 1087-1093.
- Bank, WUUW. Trends in Maternal Mortality: 1990 to 2013. Estimates developed by WHO, UNICEF, UNFPA and The World Bank. Geneva, World Health Organization; ISBN 978-92-4-1507226 (2014).
- Egbe TO, Therence ND, Gregory EH-E, Julius Aa, Boniface TN. Determinants of Maternal Mortality in Mezam Division in the North West Region of Cameroon: A Community-based Case Control Study. . International journal of tropical disease and health 15 (2016): 1-15.
- York N. EngenderHealth, Balancing the Scales Expanding Treatment for Pregnant Women with Life-Threatening Hypertensive Conditions in Developing Countries A Report on Barriers and Solutions to Treat Pre-eclampsia & Eclampsia (2007).
- Khosravi S, Dabiran S, Lotfi M, Asnavandy M. Study of the Prevalence of Hypertension and Complications of Hypertensive Disorders in Pregnancy. Open Journal of Preventive Medicine 4 (2014): 860-867.
- Ahmed NU, Rahman M, Islam MD, Ali SY, Hossain AM, Fatema Kea. Socio-demographic clinical characteristics and status of hypertension control among rural hypertensive patients. Faridpur Medical College Journal 6 (2011): 5-9.
- Mayega RW, Makumbi F, Rutebemberwa E, Peterson S, Ostenson CG, Tomson Gea. Modifiable socio-behavioural factors associated with overweight and hypertension among persons aged 35 to 60 years in eastern Uganda. PLoS one 7 (2012): 231-238.
- Leeners B, Neumaier-Wagner PK, Irawan S, Imthurn CB, Rath W. Family stability during childhood and risk to develop hypertensive diseases in Journal of Obstetrics and Gynaecology 82 (2006): 441-446.
- Mbouemboue OP, Diallo C, Marcel TT, Chantal B, Armel HNK, Jacques O, et al. A Study on Factors Related to Hypertensive Disorders in Pregnancy in Ngaoundere (Adamawa Region,Cameroon). Clinical Medicine Research 5 (2016): 6-12.
- Wiysonge SCU, Ngu BK, Mbuagbaw JN. Risk factors and complications of hypertension in Yaounde, Cameroon. Cardiovascular Journal of South Africa15 (2004): 215-219.
- O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of: a systematic overview. Epidemiology 14 (2007): 368-374.
- WH. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization (2009).
- Paudel R, Kwan L, Jitendra KS, Seok-Ju Y, Dilaram A, Rajendra K, et al. Prevalence of behavioral risk factors of cardiovascular diseases and associated socio-economic factors among pregnant women in a rural area in Southern Nepal. BMC Pregnancy and Childbirth 18 (2018): 1-9.
- Aryal K NS, Mehata S, Vaidya A, Singh S, Paulin F, Madanlal R, et al. . Non communicable diseases risk factors: STEPS Survey Nepal 2013. Kathmandu: Nepal Health Research Council (2014).
- Niraul S, Jha N, Shyangwa P. Alcohol consumption among women in a district of Eastern Nepal. Health Renaiss 11 (2014): 205-212.
- Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench 6 (2013): 14.
- Naing L, Winn T, Rusli B. Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci 1 (2006): 9-14.
- Organization, WHOWH. Obesity: preventing and managing the global epidemic. Geneva: 2000 (2000).
- Ayuk LA. Outcome of labor in preeclamptic and eclamptic patients in the maternities of the Yaoundé Central Hospital and the Yaoundé University Teaching Hospital. MD thesis (unpublished) (2002).
- Olusanya BO, Solanke OA. Perinatal outcomes associated maternal hypertensive disorders of pregnancy in a developing country. Hypertension Pregnancy Journal 31 (2012): 120-130.
- Wolde Z, Segni H, Woldie M. Hypertensive Disorders of Pregnancy in Jimma University Specialized Hospital. Ethiop J Health Sci 21 (2011): 147-154.
- Singh S, Ahmed EB, Egondu SC, Ikechukwu NE. Hypertensive disorders in pregnancy among pregnant women in a Nigerian Teaching Hospital Nigerian Medical Journal 55 (2014): 384-388.
- TabiArrey W, Akem DC, Atashili J, Mbuagbaw J, Gottlieb LM. Hypertension, an Emerging Problem in Rural Cameroon: Prevalence, Risk Factors, and Control. International Journal of Hypertension 6 (2016): 1-6.
- Kingue S, NdongNgoe C, Menanga A, Fesuh B, Nouedoui C, Muna WFT. Prevalence and risk factors of hypertension in urban areas of cameroon: a nationwide population-based cross-sectionalstudy. TheJournalofClinicalHypertension 17 (2015): 819-824.
- Amoah AGB. Hypertension in Ghana: a cross-sectional community prevalence study in Greater Accra. Ethnicity and Disease 13 (2003): 310-315.
- Adu-Bonsaffoh K, Ntumy MY, Obed SA, Seffah JD. Prevalence of Hypertensive Disorders in Pregnancy at Korle-Bu Teaching Hospital in Ghana. Journal of Gynecology and Neonatal Biology 3 (2017): 3-8.
- Duckitt K, Harrington D. Risk factors for preeclampsia at antenatal booking: systematic review of controlled studies. Biomedical Journal 330 (2005): 565.
- Shamsi U SSaNN. Epidemiology and risk factors of preeclampsia; an overview of observational studies. Al Ameen Journal of Medical Science 6 (2013): 292-300.
- Cooper DW, Hill JA, Chesley LC, Bryans CI. Genetic control of susceptibility to eclampsia and miscarriage. . Br J Obstet Gynaecol 95 (1998): 644-653.
- Suleiman AK. Risk Factors on Hypertensive Disorders among Jordanian Pregnant Women. Global Journal of Health Science 6 (2013): 1-7.
- Assis TR, Viana FP, Rassi S. Study on the major maternal risk factors in hypertensive syndromes. Arquivos Brasileiros de Cardiologia (Arq Bras Cardiol) 91 (2008): 11-17.
- Dalmáz CA, dos Santos KG, Botton MR, Roisenberg I. Risk factors for hypertensive disorders of pregnancy in Southern Brazil. Revista da Associação Médica Brasileira 57 (2011): 692-696.
- Yi Y, Jing Y, Gang Z, Weiwei X. Potential risk factor of pre-eclampsia among healthy Chinese women: a retrospective case control study. Biomedical Research 28 (2017): 1183-1188.
- Yu Y, Zhang S, Mallow EB, Wang G, Hong X, Walker SO, et al. The Combined Association of Psychosocial Stress and Chronic Hypertension with Preeclampsia. American Journal of Obstetrician and Gynecology 209 (2013): 1-16.
- Tesfaye, Abera G, Tefera, Belachew L, Sena, Belina K. Pregnancy Induced Hypertension and Associated Factors among Pregnant Women Receiving Antenatal Care Service at Jimma Town Public Health Facilities, South West Ethiopia. Journal of Gynecology and Women’s Health 10 (2018): 30-39.
- Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979-1986. American Journal of Obstetrician and Gynecology 163 (1990): 460-465.
- Adeyinka DA, Oladimeji O, Adekanbi TI, Adeyinka FE, Falope Y, Aimakhu C. Outcome of adolescent pregnancies in southwestern Nigeria: a casecontrol study. Journal of Maternal, Fetal and Neonatal Medicine 23 (2010): 785-789.
- Usta IM, Zoorob D, Abu-Musa A, Naassan G, Nassar AH. Obstetric outcome of teenage pregnancies compared with adult pregnancies. Acta Obstet Gynecol Scand 87 (2008): 178-183.
- Tessema GA, Abebe Tekeste A, Ayele TA. Preeclampsia and associated factors among pregnant women attending antenatal care in Dessie referral hospital, Northeast Ethiopia: a hospital-based study. Biomed Central Journal of Pregnancy and Childbirth 15 (2015): 1-7.
- Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MWea. First-trimester prediction of preeclampsia in low-risk nulliparous women. . Obstetrician and Gynaecology 119 (2012): 1234-1242.
- Bastani P, Hamid K, Abdollahi A. Preconception period of seminal fluid exposure and prevalence of preeclampsia in primigravida women. . Journal of Medical Science 7 (2007): 840-844.
- Adam I, Elhassan EM, MA A, Salih MM, Elbashir MI. Malaria and pre-eclampsia in an area with unstable malaria transmission in Central Sudan. Malaria Journal 10 (2011): 258.
- Shamsi U, Hatcher J, Shamsi A, Zuberi N, Qadri Z, Saleem S. A multicentre matched case control study of risk factors for Preeclampsia in healthy women in Pakistan. Biomed Central Women's Health 10 (2010): 14.
- Kiondo P, Wamuyu-Maina G, Bimenya GS, Tumwesigye NM, Wandabwa J, Okong P. Risk factors for pre-eclampsia in Mulago Hospital, Kampala, Uganda. Trop Med Int Health 17 (2012): 480-487.
- Sheldon GS. High Blood Pressure (hypertension): Does drinking alcohol affect your blood pressure? (2018).
- Yamada Y, Ishizaki M, Kido T, Honda R, Tsuritani I, Ikai E, et al. Alcohol, high blood pressure and serum y-glutamyl transpeptidase level. Hypertension 18 (1991): 819-826.
- Mishra S, Pradhan P. Association Of Maternal ABO Blood Group And Hypertensive Disorders Of Pregnancy. International Journal of Innovative Research in Technology & Science 3 (2015): 1-5.
- Zenebe W, Segni H, Woldie M. Hypertension disorders of pregnancy in Jimma University Specialized Hospital. Ethopian Journal of Health Sciences 21 (2011): 1-10.
- O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of pre-eclamsia: a systematic overview. Epidemiology 14 (2009): 368-374.
- Sibai BM, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 365 (2005): 785-799.
- Gaio DS, Schmidt MI, Ducan BB, Nucci LB, C MM, Branchtein L. Hypertensive disorders in pregnancy: frequency and associated factors in a cohort of Brazilian women. Hypertension Pregnancy 20 (2001): 269-281.
- Nisar N, Memon A, Sohoo NA, Ahmed M. Hypertensive disorders of pregnancy: Frequency, maternal and fetal outcomes. Pak Armed Forces Med J 1 (2010): 113-118.
- James M. Hypertension during Pregnancy Among Maryland Women Giving Birth 2004-2010. Maryland PRAMS Focus on Hypertension during Pregnancy 20 (2012): 1-4.
- Cho GC, Kim LY, Min KJ, Sung YN, Hong SC, Oh MJ, et al. Prior cesarean section is associated with increased preeclampsia risk in a subsequent pregnancy. BMC Pregnancy and Childbirth 15 (2015): 1-6.
- Dammavalam DSK, Kushtagi P. Pregnancy-Related Hypertension in Multigravidas with Previous Cesarean Delivery. ?stanbul Med J 18 (2017): 80-85.
- England L, Zhang J. Smoking and risk of preeclampsia: a systematic review. Front Biosci 12 (2007): 2471-2483.
- Tesfaye AG, Tilahun MR. Pregnancy Induced Hypertension and Associated Factors among Women Attending Delivery Service at Mizan-Tepi University Teaching Hospital, Tepi General Hospital and Gebretsadik Shawo Hospital, Southwest, Ethiopia. Ethiopia Journal of Health Science 2018 29 (2019): 831.
- Shikha S, Prem CS, Thimmaraju KV, Ayaz KM, Kanchan D, Biswajit D. Socio-demographic Profile of Pregnancy Induced Hypertension in a Tertiary Care Centre. Scholars Journal of Applied Medical Sciences (SJAMS) 2 (2014): 3081-3086.
- Manjunatha S, Anita K. The relationship between maternal blood group and preeclampsia International Journal of Reproduction, Contraception, Obstetrics and Gynecology 4 (2015): 1749-1752.
- May DL. Maternal blood group A and preeclampsia. Br Med J 4 (1973): 738.
- Hiltunen LM, Laivuori H, Rautanen A, Kaaja R, Kere J, Krusius Tea. Blood group AB and factor V Leiden as risk factors for pre-eclampsia: a population-based nested casecontrol study. Journal of Thromb Res 124 (2009): 167-173.
- Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. National England Journal of Medicine 346 (2002): 33-38.
- Trogstad LI, Eskild A, Magnus P, Samuelsen SO, Nesheim BI. Changing paternity and time since last pregnancy; the impact on pre-eclampsia risk. A study of 547 238 women with and without previous pre-eclampsia. International Journal of Epidemiology 30 (2001): 1317-1322.
