Preparation of Developing Biotinylated PH-Responsive Glycopolymers as Delivery Nanocarriers for Controlling Release of Bortezomib
Article Information
Ibrahim Abdalla, Zhongli Niu, Bin Sun, Xiaoze Jiang *, Meifang Zhu
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China
*Corresponding author: Xiaoze Jiang, State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China. https://orcid.org/0000-0001-7743-338X
Received: 02 July 2022; Accepted: 11 July 2022; Published: 21 July 2022
Citation: Ibrahim Abdalla, Zhongli Niu, Bin Sun, Xiaoze Jiang, Meifang Zhu. Preparation of Developing Biotinylated PH-Responsive Glycopolymers as Delivery Nanocarriers for Controlling Release of Bortezomib. Journal of Pharmacy and Pharmacology Research 6 (2022): 93-104.
View / Download Pdf Share at FacebookAbstract
To develop biotinylated pH-responsive glycopolymers and investigate their performances as anticancer drug delivery nanocarriers, bromide-terminated biotin was synthesized and then used to initiate the polymerization of D-gluconamido ethyl methacrylate (GAMA) and 2-(diethylamino)ethyl methacrylate (DEA) monomers via atom transfer radical polymerization (ATRP). Two biotinylated pH-responsive copolymers with similar degree polymerization (DP) chain segments, Biotin-P(GAMA-b-DEA) and Biotin-P(GAMA-co-DEA), were prepared by block or random architectures, respectively. The chemical compositions and self-assembly behavior of glycopolymers were characterized. The results show that the block glycopolymer self-assembles in water medium into core-shell structure assembly containing PGAMA shell and PDEA core with the 28 nm in diameter, while the random glycopolymer remains water-soluble state above pH 7.4. Both glycopolymers possess the loading ability of the BTZ with encapsulation efficiency (EE %) and loading capacity (LC %) of 83.7% and 8.4%, 78.7% and 7.9%, and the release behavior was kept at low constants with 20% and 26% cumulative amount even after 24 hours at pH 7.4, respectively. The study is expected to give the light to the development of new intelligent drug carrier system with excellent biological compatibility.
Keywords
pH-responsive glycopolymer, drug delivery nanocarrier, Bortezomib, drug loading and release
pH-responsive glycopolymer articles, drug delivery nanocarrier articles, Bortezomib articles, drug loading and release articles
Article Details
1. Introduction
Recently, the pH-responsive copolymer has attracted significant attention in the field of anticancer drug delivery systems for its spontaneous responsiveness to environment pH stimulation [1-3]. This is not only because of the simplicity of pH adjustment provides convenience to research, but also due to the design concept of carriers is based on the tiny differences between cancer cells and normal cells in pH value, namely the pH value of the normal cells is 7.4, while the pH values of the cancer cells are among 5-6.8 [4]. This kind of pH-responsive copolymer was designed as composited by pH-sensitive segment and a non-responsiveness hydrophilic segment. When the pH-responsive copolymer in a basic medium like normal physical condition, the copolymer self-assemble to core-shell structured micelles. Thus, the former segment assembles as the core to load the hydrophobic drugs for its hydrophobicity in that situation, and the later one turns into the shell to provide the stability of the entire micelle for its hydrophily. While, the pH-responsive copolymer in acidic conditions like tumor sites, the assembly demicellized to unimers to release drug as the pH-sensitive segment turn to hydrophilic.
In the current stage, reported works mainly focused on the kinds and chemical composition of the pH-sensitive segment, as well as the synthesis of monomer, investigation of polymerization conditions and controllable self-assembly behaviors [5, 6]. Among the most of pH-sensitive segment candidates, poly (2-diethylaminoethyl methacrylate) (PDEA, pKa 7.3) was a classical one as its scope of pH-induced transition of hydrophily (pH 6-8) match with the pH gap between normal cells and cancer cells, and it has been widely used in anticancer drug delivery systems [7]. In the other hand, the shell block of pH-sensitive copolymers mentioned above also played an important role in delivery properties in physical conditions, the poly(ethylene glycol) (PEG) was the most common hydrophilic segment for micelles owing to its good water solubility and biocompatibility, however it will also cause the so-called “accelerated blood clearance (ABC) phenomenon” [8], reducing the circulation time of micelles in vivo. Therefore, looking for a more biocompatible hydrophilic shell material to take the place of PEG is needed in the further clinic application.
With the further development of carbohydrate chemistry, the glycopolymer has become a kind of significant biological functional polymer due to its excellent biocompatibility, water-soluble ability, and polyhydric structure for easily modification [9-11].
Furthermore, it also became one of most suitable substitutes of a hydrophilic segment of the pH-sensitive polymer. Generally, group protection and deprotection in the polymerization of glycopolymer limited its further use, the adoption of ATRP in preparation of glycopolymer, reported in the published works [12, 13], break through this limitation and realized the direct controlled polymerization of sugar monomers [14], extending to the construction of various sugar-containing copolymer, and there are other reported works verified the advantages of glycopolymers as drug carriers [15].
Anticancer drug carriers assembled by pH-responsive copolymers loaded the hydrophobic drugs, like doxorubicin, the most studied drugs, by means of physical adsorption of hydrophobic-hydrophobic interaction or breaking covalent bonds. In recent years, BTZ as a type of polypeptide boric acid protease inhibitors has attracted attention for suitable with the chemotherapy of the malignant tumor like multiple myeloma [16]. However, it has deficiencies, such as low solubility in aqueous, relatively severe side effects, that limit its further application in clinical. The boric acid groups that bortezomib contains could complex with vicinal diol groups in the condition of pH 7, while the complexation dissociates in faintly acidic condition. This reversible complexing is available to realize the controlled release of anticancer drugs for its pH-responsive range match with the difference between normal cells and tumor cells [17-20].
With the aid of this dynamic chemical process, reported works took advantage of the dynamic complexing mentioned-above, reacting BTZ with polycatechol which contains vicinal diol, to load the drugs [21-23]. In view of the polyhydroxy structure and excellent biocompatibility the glycopolymers possessed, the glycopolymers are desired construction unit to BTZ-loading carriers and relative accurate pH-response ability, improved structural stability and biocompatibility of aforementioned carriers are available in case that glycopolymers like poly(2-gluconamidoethyl methacrylate) (PGAMA) can reversibly complex with BTZ. Hence, a kind of copolymer with different constructions contained pH-sensitive PDEA, polyhydroxy PGAMA (Biotin-P(GAMA-co-DEA) and Biotin-P(GAMA-b-DEA)) coordinated with biotin ligand, was designed to attain active targeting and controlled release in tumor sites as well as to investigate affecting regularity of drug loading and release, assemble behaviors on polymer topologies, and structural features of glyco-segment and pH-responsive segment. Furthermore, under slightly acidic pH 5.5 conditions, both glycopolymers dissolved in water as unimers, and similar accumulative release rate and release amount were acquired, the cumulative release amounts of BTZ from two glycopolymer carriers were up to 90% after 24 hours, which indicated that dynamic complexation between the carriers and BTZ plays a dominating role in drug loading and release under the circumstance.
2 Experimental Section
2.1 Materials
2-Diethylaminoethyl methacrylate (DEA, 98%) was purchased from Sigma-Aldrich Co. (USA) and purified by distilling to remove the inhibitor under vacuum. Copper (I) bromide(98%) was obtained from the Shanghai Reagent Chemical Co. Ltd. (Shanghai, China) and washed with acetic acid and ethanol, and stored under nitrogen. Trimethylamine (TEA) and N-methyl-2-pyrrolidone (NMP) were dried by anhydrous magnesium sulfate followed by distilling to purify. N, N-dimethylformamide (DMF) was distilled under reduced pressure after drying by CaH2. Bortezomib (BTZ, 98%), D-biotin (98%), N, N'-disuccinimidyl carbonate (DSC, 99%), 2-(2-aminoethoxy) ethanol (98%), 2-bromoisobutyryl bromide (98%), 2-aminoethyl methacrylate hydrochloride (95%), 2, 2’-bipyridyl (bpy, 99%), ethyl 2-bromoisobutyrate (EBiB, 98%) were obtained from a J&K Scientific Ltd (China), all other regents were used as received. The 2-gluconamidoethyl methacrylate (GAMA) monomer was synthesized according to the reference previously [13].
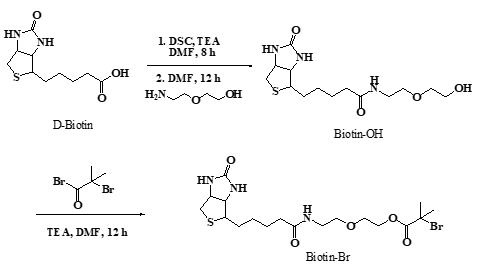
2.2 Synthesis of biotin-Br initiator
The general procedure for the preparation of the biotinylated ATRP initiator (Scheme 1) was as follows: Firstly, Biotin (5.00 g, 20.47 mmol) was activated by DSC (5.24 g, 20.47 mmol), then stirring at room temperature in the flask with TEA (3.55 ml, 24.56 mmol) and DMF (100 mL) for 8 h and followed by reacting with 2-(2′-aminoethoxy) ethanol (2.25 ml, 22.51 mmol) stirring at room temperature in flask for 12 h to form the biotinylated alcohol. After removing the solvent in vacuum, the crude product was washed by tetrahydrofuran (THF), then dissolved in methanol and precipitated in diethyl ether twice, then Biotin-OH powder was dried under vacuum overnight (3.10 g, 46% yield).
The esterification of biotinylated alcohol (0.80 g, 2.42 mmol) was carried out by reacting with 2-bromoisobutyryl bromide (0.3 mL, 2.66 mmol), mediated by TEA (0.42 mL, 2.91 mmol) and diluted by DMF (10 mL), in the ice-water bath with stirring about 12 hours, then the solvent was evaporated under vacuum to obtain the solid crude product, the product was dissolved in 25 mL of chloroform, washed three times with saturated sodium bicarbonate, brine and dried with anhydrous magnesium sulfate. Then the mixture was filtered to obtained pale-yellow liquid and the solvent was evaporated to obtain the product (1.68 g, 25%yield).
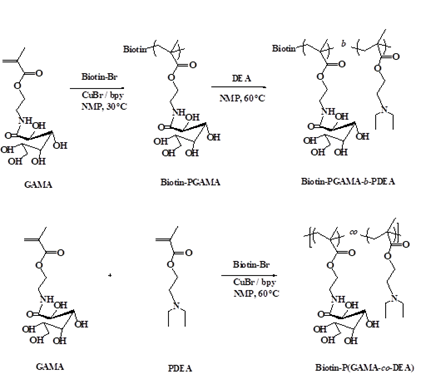
2.3 Synthesis of pH-sensitive glycopolymers biotin-P(GAMA-co-DEA) and biotin-PGAMA-b-PDEA
The preparation of glycopolymers is initiated by biotin-Br via atom transfer radical polymerization (ATRP) under the nitrogen condition (Scheme 2). The initiator biotin-Br (0.0312 g, 0.065 mmol), bpy (0.0203 g, 0.130 mmol) and GAMA monomer (0.50 g, 1.630 mmol) were dissolved in 2 mL NMP in a flask and deoxygenated by bubbling N2 at room temperature about 30 min before adding the catalyst CuBr (9.30 mg, 0.065 mmol) into the flask, taking samples after reacting 5 h at 30ºC, the monomer conversion rate was characterized as more than 95% by 1H NMR. Then the deoxygenated DEA (0.40 mL, 1.950 mmol) was injected into the flask and the mixture was reacted at 60ºC about 24 h before stopping the reaction by cooling with liquid nitrogen and yield Biotin-P(GAMA-b-DEA) (0.56 g, 63%) after dialysis against water for 2 days and freeze-dried.
The Biotin-PGAMA-b-PDEA was prepared by the same procedure replacing biotin-Br by EBiB (10 µL, 0.065 mmol) and yield 0.51 g, 59%. Furthermore, the random Biotin-P (GAMA-co-DEA) was obtained by adding monomers simultaneously and reacted in 60°C about 24 hours and yield 0.67 g, 75%.
2.4 Self-assembly and BTZ loading of glycopolymers
Biotin-PGAMA-b-PDEA (20 mg) or Biotin-P(GAMA-co-DEA) (20 mg) were dissolved in acidic water and adjusted the pH to 5.0 and 9.0 respectively to prepare four samples (10 mL) provided with a polymer concentration of 2 mg/mL. The drug-loaded carriers were prepared by means of dialysis method under pH 9.0 conditions with 0.040 g Biotin-PGAMA-b-PDEA or Biotin-P(GAMA-co-DEA) each as well as 0.008 g BTZ, the glycopolymer and BTZ were dissolved in 2 mL DMSO first, then 10 mL of aqueous solution at pH 9 was added dropwise to induce micellization before dialyzed against distilled water using dialysis membrane (MWCO 3500 ) and shielded from light for 24 hours.
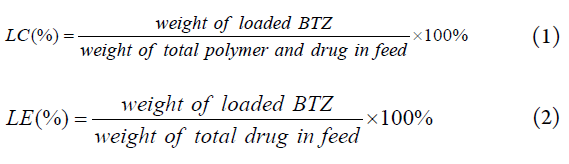
The BTZ concentration was measured by a UV-Visible spectrophotometer (UV-Vis, TU-1901 Beijing spectrum analysis of general instrument Co., Ltd) at 260 nm according to the calibration curves of different concentrations of BTZ in DMSO analyzed previously. The loading content (LC%) and loading efficiency (LE%) of BTZ were calculated using equation (1) and (2).
2.5 In vitro BTZ release of pH-responsive glycopolymer carriers
The drug release behavior of the BTZ-loaded carrier, Biotin-PGAMA-b-PDEA and Biotin-P(GAMA-co-DEA), was investigated under pH 5.5 and pH 7.4 PBS (phosphate buffered saline) buffer (0.01 M) medium via diafiltration method at a low total concentration of 16.7 μg/ml at 37.5°C. The drug-loaded micelles PBS solutions (5 mL, 1 mg/mL) dialyzed against 45 mL PBS solutions in the dialysis membrane (MWCO 3500 Da) in an incubator. The PBS (5 mL) was added after sampling the medium (5 mL) outside the membrane at specific time intervals. The accumulative release percentage of BTZ were calculated using equation (3), and all release experiments were carried out in triplicate, and all the results were reported as mean ± standard deviation (n=3).
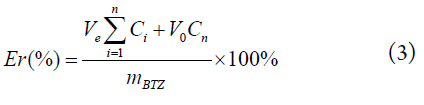
where V0 is the volume of medium (50 mL), Ve is the volume of sampling, Ci is the BTZ concentration of the ith sample, and mBTZ is the weight of the total drug in the dialysis bag.
3. Characterization
The copolymers and biotin initiator were dissolved in D2O or CD3OD, respectively, and characterized by 1H NMR spectra with Avance 600M NMR (Bruker, Switzerland). The sizes of assemblies were determined by Dynamic lights scattering (DLS) measurement with a ZETA-SIZER Nano Series Nano-ZS (Malvern Instruments Ltd., UK) to characterize the particle sizes and size distribution thereof at different pH conditions. The samples were prepared at 1 mg/mL concentration and removed dust by filtered using a 0.45 um filter membrane before measurement. The size and size distribution of micelles were recorded by testing samples via fifteen runs and each run keeps 10 seconds, every sample was measured three times and the size was averaged. The scattering angle is 173° for presenting size distribution data. The absorbance at 260 nm corresponding to the concentration of BTZ was measured by a UV-Visible spectrophotometer (UV-Vis, TU-1901 Beijing spectrum analysis of general instrument Co. Ltd., China) to describe the loading efficiency and profile of the drug release.
4. Results and Discussion
4.1 Synthesis and characterisation of initiator biotin-Br
In the research on the treatment of cancer by polymeric nanocarriers, polymeric nanoparticles as one of most popular nanocarriers are usually required to be swallowed only by cancer cells to achieve the controlled release of anticancer drugs within the cancer cells to avoid side-effect of the anticancer drug [24, 25]. Therefore, varies of cancer cell overexpressed factor or functional group receptor has been widely used in the anticancer drug delivery systems. Among them, biotin as a kind of ligand readily available but with high affinity to the biotin receptor that overexpressed at the surface of many kinds of cancer cells has attracted much attention [26]. The most common and convenient method introduces the biotin group to the termination of the polymer is to adopt biotin as the initiator to initiate the polymerization.
With the rapid development of living free radical polymerization, ATRP has been confirmed as applicable to controllable polymerization of a large amount of functional monomer for its mild polymerization conditions, simple procedures and protection of monomers functionality [27]. There are reported works synthesized biotinylated initiator successfully through activated the carboxyl of biotin and amidated with molecule activation before esterifying with a carboxylic acid to obtain biotin terminated ATRP initiator [28, 29].
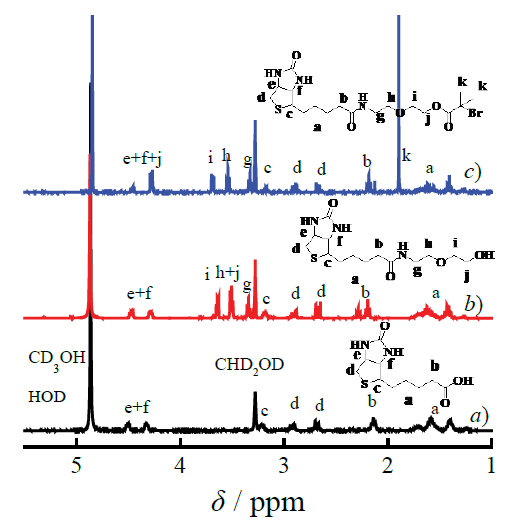
In order to increase esterification efficiency, the initiator biotin-Br was synthesized by a sequential process of amination and esterification of the D-biotin with dihydric alcohol and acylbromide rather than esterifying with a carboxylic acid. The 1H NMR was used to confirm the chemical composition and constructions of the intermediate compound biotinylated alcohol and the initiator biotin-Br. The spectra of 1H NMR showed the different characteristic signals based on the introduction of the dihydric alcohol (δ 3.65, 3.53 and 3.36 ppm, as shown in Figure 1 (b)) compared with biotin (as shown in Figure 1 (a)), furthermore the initiator biotin-Br (as shown in Figure 1 (c)) was characterized by the 1H NMR for the characteristic signals appeared at 1.80 ppm related with the Br introduced [30, 31]. Moreover, the ratio of the area of the peak (δ 1.80 ppm) to the area of the signal (δ 3.27-3.20 ppm) was 1:6, consistent with the results reported, which further defined the chemical structure of the initiator.
4.2 Synthesis and characterization of glycopolymers
For the sake of investigating novel polymeric nano-carriers for delivering drugs, pH-sensitive glycopolymer contained PGAMA, suitable substitution for PEG for its excellent water solubility and biocompatibility, and pH-sensitive PDEA, which response interval is at pH 6-8, were synthesized via ATRP. For the preparation of polymers contained PDEA segments, the ATRP is usually selected carried through in a protic solvent (such as methanol or isopropanol) [32]. However, the solubility of GAMA in the protic solvent was low, thus NMP was chosen to replace the methanol or isopropanol as the solvent to carry on the polymerization for the GAMA has better solubility in NMP. Glycopolymers Biotin-P(GAMA-co-DEA) and Biotin-P(GAMA-b-DEA) were prepared by ATRP and the construction of block- and random- was controlled by the addition timing of the monomers. It needs to note that in the polymerization of the block copolymer, the GAMA monomer conversion rate was monitored as over 95% before adding the second monomer DEA.
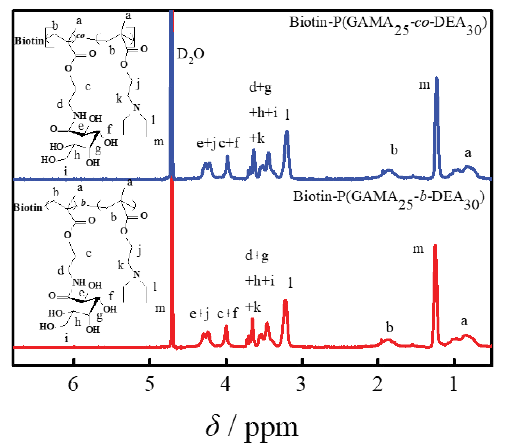
Purified glycopolymers with different construction were characterized by 1H NMR as shown in Figure 2. The result showed the characteristic signals of DEA (δ 1.23 and 3.20 ppm) and GAMA (δ 3.50-4.50ppm) and the ratio of the area of representative peaks confirmed the ratio of the degree of the DEA and GAMA was 30:25. According to the conversion rate of GAMA was over 95%, and designed DP of GAMA was 25, the glycopolymer mentioned above expressed as Biotin-P(GAMA25-co-PDEA30) and Biotin-P(GAMA25-b-DEA30). For the signal coverage of the GAMA, the characteristic signals of the biotin appeared in δ 1.8-2.0 ppm only. Additionally, the GPC results of Biotin-P(GAMA25-co-PDEA30) and Biotin-P(GAMA25-b-DEA30) both showed as a singlet, the Mn were 9630 and 10568, Mw/Mn were 1.20 and 1.21, respectively, which further indicated the successful synthesis of those glycopolymers.
4.3 Assembly formation of pH-responsive glycopolymers
For the traditional polymer nano-carrier system, it is necessary to assemble into shell-core structured nano-aggregate spontaneously in pure water solution without an organic solvent, thus loading the drug via hydrophobic-hydrophobic interaction. The pH response behavior of PDEA and PDEA contained block polymers has been studied extensively, as the PDEA segment protonated and showed hydrophily in acidic condition, while deprotonated and showed hydrophobicity in basic condition. On the contrary, the PGAMA segment was water-soluble in whole pH range without pH responsiveness. In theory, the pH-responsive block glycopolymer would self-assemble into shell-core structured nano-aggregate induced by pH change while the self-assembly behaviors of random glycopolymer further study were needed.
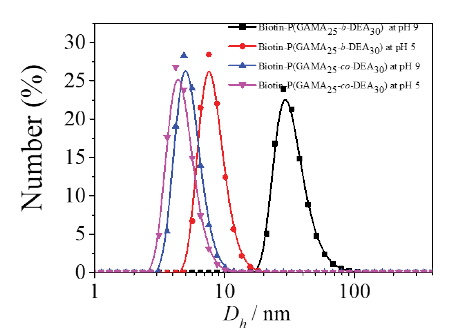
Assemblies were formed from pH-sensitive glycopolymers Biotin-P(GAMA25-co-PDEA30) and Biotin-P(GAMA25-b-DEA30) in aqueous solution at different pH values. The results of dynamic light scattering (DLS) measurement showed that the sizes of two copolymers changed at different pH value as presented in Figure 3, wherein the size of random glycopolymer altered slightly from 4.2 nm to 4.8 nm when the pH changed from 5 to 9, which indicated that the random copolymer was not assembled as core-shell structured micelle at that condition resulted by the enhancement of the entire hydrophilia of random chain for the outstanding hydrophily of the GAMA contained and the similar molar number of the GAMA and DEA. This is the same as the conclusion of reported works that the embedded water-soluble segment declined the responsive assemble ability of responsive segment [33]. There against, the block copolymer showed a remarkable alteration (7.5 nm to 28.2 nm) when the pH changed at the same condition like the random one, which indicated that the block copolymer formed as micelle which possessed two parts: the hydrophilic shell formed by the PGAMA, the pH responsive core formed by the deprotonated PDEA, which is consistent with that reported by Liu and our works [25, 34, 35].
4.4 The in vitro BTZ loading of pH-responsive glycopolymers
The drug-loaded carriers were prepared by means of dialysis method under pH 9.0 conditions and further measured by the double-beam UV-Vis Spectrophotometer at a relevant wavelength (296 nm) to describe the loading efficiency cooperate with BTZ absorbance standard curves. Moreover, calculated the EE% and LC% of Biotin-P(GAMA25-co-PDEA30) were 83.7% and 8.4%, and the EE% and LC% of Biotin-P(GAMA25-b-DEA30) were 78.7% and 7.9%, on the basis of formulas listed in the experiment part.
The BTZ as a type of polypeptide boric acid protease inhibitors has attracted much attention for its special efficacy of multiple myeloma. However, BTZ showed poor water solubility, even when its boric acid group ionization partially at basic condition, which limited its further application in the clinic. Theoretically, the hydrophobic BTZ load into the carriers assembled by glycopolymers mentioned above through hydrophobic-hydrophobic interaction. Nevertheless, the influence of poor assemble capacity of Biotin-P(GAMA25-co-PDEA30) on drug load capacity was small, which indicated that the complexation between GAMA and BTZ become the dominant mechanism in drug loading, corresponding with reported works which clarified the complexation between glycopolymer and BTZ [36].
4.5 In vitro BTZ release Behaviors
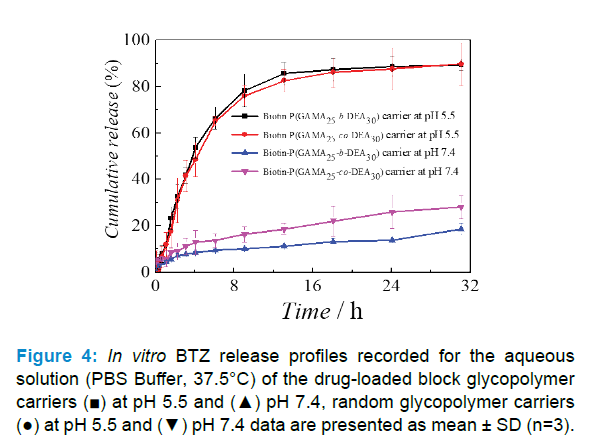
The drug release behavior of the BTZ-loaded carriers was investigated in PBS buffer with different pH value at 37.5 °C after dissolving the drug-loaded carriers. UV-Visible spectrophotometer was used to characterize the BTZ cumulative released quantity of samples to obtain the release profile of BTZ as shown in Figure 4.
The results showed that BTZ-loaded carriers possessed pH responsibility, wherein the 24 hours cumulative release amount of block copolymer carrier was less than 20% at pH 7.4 but come near 90% at pH 5.5, and of random copolymer carrier was 26% at pH 7.4 but come near 90% at pH 5.5, which indicated that two types of copolymers were provided with controlled release ability, namely the copolymer-assembled carriers would show a prompt release in mild acid condition like tumor site and would maintain a low-level premature release in alkalescence condition similar with normal cells [37, 38]. Moreover, the results further disclosed that the block one outperforms random one at stability and controlled release in two conditions. Combined with loading results and the assemble abilities of two copolymers, the drug loaded in random one exposed to the aqueous for the carrier structure which lead more premature release at pH 5.5 and inferior controlled release performance, which further confirmed that the loading and release of BTZ mainly controlled by the revisable complexation between GAMA and BTZ.
5. Conclusion
The ATRP initiator biotin-Br was synthesized from Biotin, and the two types of glycopolymers Biotin-P(GAMA25-co-PDEA30) and Biotin-P(GAMA25-b-DEA30), with different construction but same chemical component were synthesized by using the ATRP method. This study shows that assemblies based on PGAMA and PDEA copolymers load and release the BTZ in an efficient way in which the dynamic complexation play the main role, wherein the block copolymer shows a better-controlled release performance for its micelle construction, and the revisable complexation between GAMA and BTZ afford a new thought for the design of the BTZ carriers.
Acknowledgment:
The authors gratefully acknowledged the financial support of the National Natural Science Foundation of China (No. 51473035 and No. 21204010), the Research Program of Shanghai Science and Technology Commission (No. 13NM1400102 and No. 14521100600), and the Fundamental Research Funds for the Central Universities (No. 2232014D3-35).
Conflicts of interest
The authors declare no conflict of interest.
References
- Manchun S, Dass CR, Sriamornsak P. Life Sci 80 (2007):381-387.
- Bajpai AK, Shukla SK, Bhanu S, Kankane S. Polym. Sci 33 (2008): 1088-1118.
- Zhanguang C, Tianhe S, Yurui, P, Xi C, Junhui C, Guomin Z, Sihua Q. Analyst 136 (2011):
- Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z. Biomaterials 85 (2016):152-167.
- Kocak G, Tuncer C, Bütün V. Chem. 8 (2016): 144-176.
- Dai S, Ravi P, Tam KC. Soft Matt. 4 (2008): 435-449.
- Tang Y, Liu SY, Armes SP, Billingham NC. Biomacromolecules 4 (2003):
- Ishida T, Kiwada H. J. Pharm. 354 (2008):56-62.
- Becer CR. Rapid Commun. 33 (2012): 742-752.
- Ladmiral, V.; Melia, E.; Haddleton, D. M., Polym. J 40 (2004):431-449.
- Li X, Chen G. Chem. 6 (2015):1417-1430.
- And RN, Armes SP. Macromolecules 36 (2003): 4675-4678.
- Narain R, Armes SP. Biomacromolecules 4 (2003):
- Auzély-Velty R, Cristea M, Rinaudo M. Biomacromolecules 3 (2002):
- Ahmed M, Mamba S, Yang XH, Darkwa J, Kumar P, Narain R, Bioconjugate Chem. 24 (2013): 979-986.
- Groll M, Berkers CR, Ploegh HL, Ovaa H. Structure 14 (2006): 451-456.
- Su J, Chen F, Cryns VL, Messersmith P,B. Am. Chem. Soc. 133 (2011):11850-11853.
- Pasparakis G, Cockayne A, Alexander C. Am. Chem. Soc. 129 (2007): 11014-11015.
- Li Y, Xiao W, Xiao K, Berti L, Luo J, Tseng HP, Fung G, Lam KS. Angewandte Chemie International Edition 51 (2012):2864-2869.
- Taketani A, Andriana BB, Matsuyoshi H, Sato H. Analyst 142 (2017).
- Wang M, Yu W, Ke H, Shao N, Cheng Y. Sci. (2015).
- Wang M, Cai X, Yang J, Wang C, Tong L, Xiao J, Li L. ACS Appl. Mater. Interfaces.
- Shen J, Song G, An M, Li X, Wu N, Ruan K, Hu J, Hu R. Biomaterials (2014):316-326.
- DU JinZhi, DU XiaoJiao, MAO ChengQiong, Wang J. Am. Chem. Soc. (2011):17560-17563.
- Abdalla I, Xu J, Wang D, Tong H, Sun B, Ding B, Jiang X, Zhu M. RSC Adv. 9 (2019):1814–1821.
- Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Soc. Rev. 42 (2013):1147-1235.
- Matyjaszewski K. Macromolecules 45 (2012):4015-4039.
- Qi K, Ma Q, Remsen EE, Jr CC, Wooley KL. Am. Chem. Soc. 126 (2004):6599-6607.
- Bontempo D, Li RC, Ly T, Brubaker CE, Maynard HD. Comm. 37 (2005):4702-4704.
- Corcoran O, Lindon JC, Hall R, Ismail, IM, Nicholson JK. Analyst 126 (2001): 2103-2106.
- Jochen K, Murray C, Horst K, Analyst 132 (2007): 692-705.
- Xiaoze J.; Shizhong L.; S. P. A.; Wenfang S., A.; Shiyong L., Macromolecules 39 (2006): 5987-5994.
- Zschoche S, Rueda J, Boyko V, Krahl F, Arndt KF, Voit B. Chem. Phys. 211 (2010): 1035-1042.
- Liu SY, Jvm W, Tang YQ, Billingham NC, Armes SP, Tribe K. Macromolecules 35 (2002):6121-6131.
- Liu S, Weaver JVM, Save M, Armes SP. Langmuir 18 (2002): 1347-1353.
- Striegler C, Schumacher M, Effenberg C. Müller M, Seckinger A, Schnettler R, Voit B, Hose D, Gelinsky M, Appelhans D. Biosci. 15 (2015): 1283-1295.
- Shan X, Mao J, Long M, Ahmed KS, Sun C, Qiu L, Chen J. Appl. Polym. Sci. 136 (2019):47854.
- Wang X, Sun X, Jiang G, Wang R, Tao W. Appl. Polym. Sci. 128 (2013): 3289.