Primary Prevention of Cardiovascular Disease with Collaborative Diabetes Study in the Efficacy of Statins: Meta-Analysis of Randomized Controlled Trials
Article Information
Jiang Hong, Hassah Batool Iftikhar*
Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, PR China
*Corresponding Author: Dr. Hassah Batool Iftikhar, Department of Cardiology, Renmin Hospital of Wuhan University, 430060, Wuhan, Hubei Province, PR China
Received: 08 May 2018; Accepted: 11 July 2018; Pblished: 19 July 2018
Citation: Jiang Hong, Hassah Batool Iftikhar. Primary Prevention of Cardiovascular Disease with Collaborative Diabetes Study in the Efficacy of Statins: Meta-Analysis of Randomized Controlled Trials. Cardiology and Cardiovascular Medicine 2 (2018): 123-134.
View / Download Pdf Share at FacebookAbstract
Context: A recent meta-analysis demonstrated the use of statin therapy in an association of ischemic conditioning of high-risk T2DM development.
Objective: The purpose of this research is to investigate the effectiveness of statin therapy in metabolic cardiovascular syndrome and mortality by meta-analyses of longitudinal studies.
Data sources: We identified relevant trials in a literature search of PUBMED, MEDLINE, EMBASE and Cochrane Central Register from 1994-2013 obtaining the unpublished data to exist the useful finding of statin therapy. Study selection: We included the randomized controlled end points by checking the reference lists of previous systematic reviews conducted on1000 participants per year updated on April 2018 with the clinical follow up of least treatment and titrating dosing issues in measuring the risk ratio of restricted events with the random effect of meta-analysis.
Data extraction: The selected studies for inclusion describe the characteristics of T2DM incidence experiencing the outcomes of fatal/nonfatal myocardial Infarction, stroke, left ventricular ejection fraction, glycated hemoglobin, hypertension history and changes in LDL-C and TOT-C the all-cause mortality changes in the quality life. We observed the specific relative risk and mean difference in the combined random effects of statistic heterogeneity assessment. We also contacted the trial authors to obtain the missing data.
Results: In 29 study trials with the participants of 3000 developed diabetes representing the additional cardiovascular events groups per 1000 patients yearly experience (3135-3165) respectively with an over-weighted mean STD follow up of 5 years (IV Random 0.43; Confidence Interval -0.88, 1.75 I2=97%) and T2DM on receiving intensive-dosing (IV Random 0.29; Confidence Interval -4.64,
Keywords
Cardiovascular Disease; Myocardial Infarction; Hypercholesterolemia
Article Details
1. Introduction
Cardiovascular disease is a well-known predictor of metabolic syndrome and atherosclerosis varying the impact of conventional risk factors substantially disables the world budgets. Based on the act of statin therapy in the culmination of adverse events with the history of diabetes, recall further the use of lipid- lowering agents in reducing the established co-morbidity. A recent Meta-analysis of 13 RCTs of standard involving the T2DM leading cause of probable death implicates the risk of health prevention by providing the ambiguous included studies reliable to evaluate the treatment of coronary and cerebrovascular disease. The challenging catastrophic of sudden death incidence target the large population to diagnose the criteria of screening dyslipidemia disorders and the role of glycated hemoglobin for selecting the intercept measures on the prevailing predisposing glycemic alterations. Moreover, the standard goals of calculating the incidence are to estimate the basic effects of pro-inflammatory mediators, fatal/nonfatal ischemia, hypertension, stroke, heart failure, and hypercholesterolemia.
According to the publication of JUPITER study justify the current issue of statin drugs use in the emergence of extreme cases reinforcing the acute myocardial infarction subjects on the premise of LDL, HDL, TG, BMI, and FPG prospecting the concept of less vulnerability and efficacies conflict the absolute risk of placebo comparative studies [1-3]. We included the studies for conducting randomized trials on statin risk ratio of preventions in the prevalence of CHD and occurrence of CVD risk factors observing the adjustments of ages, sex and correlated cholesterol levels in the patho physiological conditions of insulin resistance to compute the epidemiological distinct numerated dysfunctions in upcoming years worldwide.
2. Methods
In using the initial methods, the various numbers of studies determined the scope of randomized endpoints of statin therapy in the preventions of T2DM perceives the key questions for this review. The disclosures of intervention follow up subcategory the placebo authentic tests in the ascertainment prior to safety cautious outcomes of CV risk factors. A research on Wiley Online Library, PUBMED, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (from 1991), and Cochrane database of systematic reviews from 2005 to 2016 identify the aggressive performances of intensity in old adult cases updated on initial 2010-2011 with the published figures of 2
readers (D.P. and P.W) in 1996 reviewed a settled discrepancies. After the preparation of the manuscript, it’s been peer reviewed as an unpublished draft for the public comments in the evaluation of efficacies and effectiveness in the particular reduction of cholesterol.
2.1 Data sources and searches
According to the caliber data query, the specific subgroups in the ratio of high risk developing diabetes stage II, We investigated the all the lab reference ranges in the collection of values for the notable events with the associated medians on receiving the drugs.
2.2 Quality assessment
To evaluate the independent results of quality based characteristics in treatment allocate the authors used tool [4] to differentiate the similarity and dissimilarity between the eligible subgroups. Therefore, in the approximation treatment rate availability respective to blind analysis on each trial thereby need an awarded Delphi score rating of 0-9 in the resolved consensus of discussion.
2.3 End points
At the consideration of non insulin dependent diabetes with an high developing risk factors eventually recognize the commenced medication on trials with greater values of fasting glucose values, We performed the criterion of required reanalysis by including the nonstandard sensitivity to the study composite to cardiovascular death ratio, myocardial infarction high risk, ischemia or the pharmacological interventions leading the all-cause mortality. As an alternative of replenishing shorter cases, a large number of acute coronary syndrome conditions with prespecified indexed events measures the procedures of randomization driven by other cardiac-related diseases.
2.4 Statistical analysis
In the potential associations of mild to moderate dose statin therapy, we pinpointed the hazard ratio on diabetes onset with adverse events, we calculated the odd ratio (or) and 95% CI from the baseline started developing risk factors in controlling the major risk follow up of least 1 year. The pooled effect model of Meta-analysis accounted the heterogeneity with std mean difference to introduce the newly distinguishable method subjecting the evidence- based trial data across the attributes of statistic variation proportions asses the publication bias through funnel plots. While comparing the results on the extraploratory analysis of random effect, we read the P value respective to the regimen in the significance of Review Manager 5.0 and Stata version 10.1.
3. Results
Based on 5 randomized clinical trials, the combined data of STD mean differences with the follow up of cardiac- related symptoms in participants favor the statin therapy according to the experimental study. As summarized in Figure 1 and Figure 2 relate the outcome results on absolute relative risk corresponding to the significance of major diabetic leading events value the beneficial investigations in demonstrating the comparable method of each subgroup.
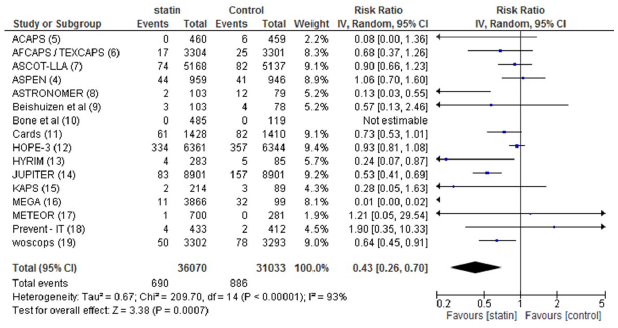
Figure 1: Over all meta-analysis of comparative studies in statin use and risk ratio of all cause mortality events.
3.1 Cardiovascular outcomes
On the referred studies based outcome result the control of risk factors with an overall effect of CVD events (IV random ? 0.43; 95% CI ?0.88, 1.75) heterogeneity 3.92; P=0.52; I2=97%, Heart Failure (IV random ? 0.33; 95% CI
?0.34, 0.99) heterogeneity 0.15; P= 0.34 I2=41%, Coronary Artery Disease (IV random ? 0.14; 95% CI ?0.65, 0.38) heterogeneity 0.17; P=0.61; I2=69%. The standard mean difference (SMD) funnel plot on each study trial notice the asymmetry in the illustrative simulations to influence the visual analytical detection by using empirical study data. The effect size in primary studies precise the false-positive results by measuring the reliable end points to conclude the sample size precision in the combined variations.
3.2 Outcome of T2DM
The assigned cases in the incidence of diabetes on receiving therapy (IV random ? 0.29; 95% CI ?4.64, 4.06) in the absolute terms, of significance shows the sources of heterogeneity 14.22; P=0.90; I2=98%. Likewise analysis of publication bias evidence.
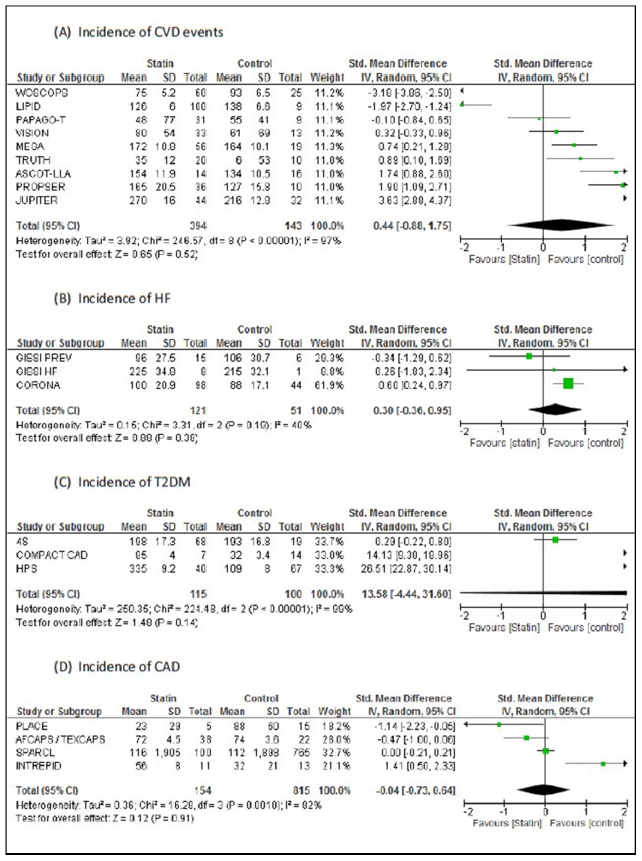
Figure 2: (A) Forest plot standard mean difference evaluating statin therapy in the incidence of CVD events; (B) Incidence of heart failure; (C) Incidence of type 2 diabetes mellitus; (D) Incidence of coronary artery disease. Meta- analysis was performed using a random-effect model. CI= confidence interval; STD = standard mean difference.
3.3 Subgroup analysis
The consistent study characteristics as shown in Table 1 define the study of study design preceding the duration strategy prior to adults and old age group in randomizing the effect. As we discussed the median variables of cholesterol levels, glycated hemoglobin and glucose concentration the study provided different classes of drugs in the normal resistivity conditions with case-control studies.
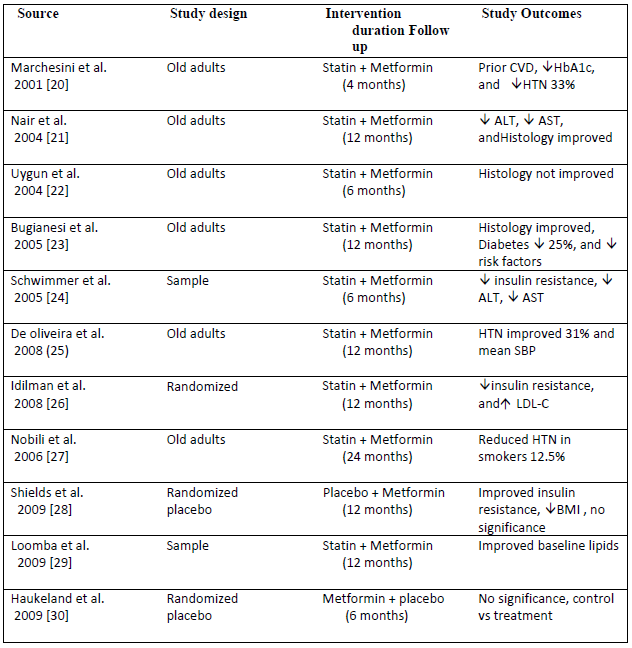 |
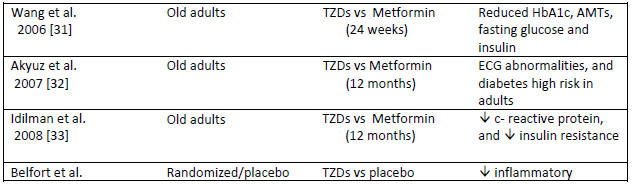 |
Table 1: Characteristic of included studies in clinical trials of statin vs placebo.
3.4 Trial sequential analysis
According to the qualitative studies, TNT [15] and IDEAL [16] the SSMD can be used on the fixed effects model if it produced the same results of randomized control Meta-analysis with onset pooled data. Mainly the aim is to achieve the expected consumption of controlling factor in preventions of CVD risk factors and additionally diabetic hypertension in the association of vascular disorders. Thus on calculating the hazard ratio on variance analysis the aim of nonstandard pharmacological drugs on a specific regimen in the correlation of cholesterol levels must evaluate the co-variable regression independently with the protocols of bringing better results on Randomized Controlled Trials.
4. Discussion
The present-day Meta-analysis affected the statin dosage of 50mg-80mg/day in several clinical trials discern the manifestation of CHD (1.112, 95%CI 1.04-1.22) [49] with a recurrent rhabdomyolysis in adults stated by American guidelines [12,13]. The interesting mechanistic phenomenon in the assessment of statins effectiveness possibly bring-up the unmatched statistical differences in controlling the underutilized insulin resistance with the progression of various consecutive dysfunctions sensitizing the immune cells in vitro [50, 51]. Therefore, the unknown evidence on literature reviewing 1000 patients/year of baseline recheck the drugs beneficial effect since 2004 [9] to scope the decreasing outcomes of adherence in terms of traditional risk factors as mentioned in fewer studies of fixed effect.
The American Health Care providers established a study treatment of statin drugs in the match of propensity score selecting the basic characteristic of new cases at potential risk on diabetic restrictions of HR (0.63, 95% CI 0.25- 1.60). The affirmed outcomes on making knowledge gap empty exacerbate the complications of sudden death in maintaining the worsening of glycemia intensification. Regardless, the use of combined therapy including ACEIs or ARBs and ? blockers or diuretics alleviate the potency on findings in each subgroup with the demographic results of high CRP, GI disturbances, disturbed lipid levels and cancer symptoms. Therefore, the survival bias on large analysis explains the confounding factors in the average modified conditions revealing the conditions of obesity and advanced aging. According to the following principles of Swerdlow et al. [9] examined the study of genetics which strongly concluded the amount of titrated dosing strike numerous micro-vascular and macro-vascular diseases on the
sites of skeletal muscles in the association of neurological disorders. The other studies of pharmacokinetics exposure to variability compose the IHD adequately in the prolonging T2DM diminish the homeostatic regulations of glucose activity, mitochondrial dysfunctions, beta cells death and metabolic blockade releasing the behavior of lipophilic state.
The recent US food and drug administration declare statins to be the first choice therapy by the majority of recommended physicians in the prevalence of adulthood diabetes onset irrespective of cardiac diseases. And also the designed National Health Insurance extrapolated the prospective and retrospective studies on Asian vs Americans clinical conditions similarity to verify the age reflux, social history, and multiple disorders on record of sampling cohort studies in making easier balanced population-based decisions accurately. Moreover, the published projects enrolled a large group of females with T2DM as follows MEGA, ASFA, TNT, IDEAL, SEARCH and JUPITER study trying atorvastatin and simvastatin with no appearance of current vascular disease ensure the morbid analysis on the strong inference of time duration curative symptoms. As also reported by PROPSER and WOSCOPS the results in the concerns of protection noted the reduction of malignancies deriving the uncertain side effects of myopathy and hepatotoxicity. At the examination of fibrinolytic carried out for decades with placebo testing reveal the vigilance of statins directly explaining the histology of cells and insulin action which already been attested on animal models to strengthen the relevance of powerful detection majorly in the intensive drugs bias evidences. Hence, altogether the negative and beneficial effects neutralize the glucose tolerance on the referred robust trial of (J-PREDICT) study in 2005. But still further to understand the clinical implications, the interpretations of previous studies are needed with several newly clinical diagnostic criteria for the authorized bias. In conclusion, the extended analyses about earlier findings require a perception of cholesterol observation in treating diabetogenicity to precede the subsequent life style modifications.
5. Limitations of Study
As already mentioned in the recruit exclusion of CVD related symptoms minimize the proportional outcomes of diabetes attribute the involvement of dyslipidemia with the critical magnitude of LVH. However, the guidelines respective to equal results no highly influenced the elderly as seemed earlier in the panels of collective data. The involvement of heterogeneity in risk ratio reduction across the ranges of increased survival rates on annual rates of 20%-30% with the stable CAD events in the consideration of PEACE study. But the contemporary significance of pooled study data in >65-year ages assumed an obvious high risk constitute describing a poor scoring system. Thus, the correct assessment of prognosis needs a long-term statin drugs for the auxiliary improvements in future in cardiovascular based on predictions at both the genders of identification in not denying the favorable experiments.
6. Conclusion
The Scientific literature demonstrates the consistent variations of each component generalized the contributable cardiovascular events in association of diabetes modified biological experimental statins in context of statistics. The importance of cholesterol lowering still unclear the analysis of insulin hypothesized complications in the detection of potential effect. Therefore, in the future necessarily to recognize the high risk outcome in comparative studies, the excluded studies must be added in the conduction of detection bias to clarify the differentiation of effective result.
Conflicts of Interest Statement
The author declared that they have no competing interest.
Acknowledgement
The Author would like to thank Dr. Hassam Ali for the great help in revising the manuscript for important intellectual content.
References
- Cornell JE, Mulrow CD, Localio R, et al. Random-effects meta-analysis of inconsistent effects: A time for change. Ann Intern Med 160 (2014): 267-270.
- Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry inmeta-analyses of randomised controlled trials. BMJ 343 (2011): d4002.
- Furberg CD, Adams HP Jr, Applegate WB, et al. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Circulation 90 (1994): 1679-1687.
- Knopp RH, d’Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of coronary heart disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care 29 (2006): 1478-1485.
- Furberg CD, Adams HP Jr, Applegate WB, et al. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Circulation 90 (1994): 1679-1687.
- Downs JR, Clearfield M, Weis S, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA 279 (1998): 1615-1622.
- Sever PS, Dahlöf B, Poulter NR, et al. ASCOT Investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Lancet 361 (2003): 1149-1158.
- Chan KL, Teo K, Dumesnil JG, Ni A, Tam J ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 121 (2010): 306-314.
- Beishuizen ED, van de Ree MA, Jukema JW, et al. Two-year statin therapy does not alter the progression of intima-media thickness in patients with type 2 diabetes without manifest cardiovascular disease. Diabetes Care 27 (2004): 2887-2892.
- Bone HG, Kiel DP, Lindsay RS, et al. Effects of atorvastatin on bone in postmenopausal women with dyslipidemia: a double-blind, placebo-controlled, dose-ranging trial. J Clin Endocrinol Metab 92 (2007): 4671-4677.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364 (2004): 685-696.
- Yusuf S, Bosch J, Dagenais G, et al. HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 374 (2016): 2021-2031.
- Anderssen SA, Hjelstuen AK, Hjermann I, Bjerkan K, Holme I. Fluvastatin and lifestyle modification for reduction of carotid intima-media thickness and left ventricular mass progression in drug-treated hypertensives. Atherosclerosis 178 (2005): 387-397.
- Ridker PM, Danielson E, Fonseca FAH, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359 (2008): 2195-2207.
- Salonen R, Nyyssönen K, Porkkala E, et al. Kuopio Atherosclerosis Prevention Study (KAPS): A population- based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 92 (1995): 1758-1764.
- Nakamura H, Arakawa K, Itakura H, et al. MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA study): a prospective randomised controlled trial. Lancet 368 (2006): 1155-1163.
- Crouse JR III, Raichlen JS, Riley WA, et al. METEOR Study Group. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA 297 (2007): 1344-1353.
- Asselbergs FW, Diercks GFH, Hillege HL, et al. Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVEND IT) Investigators. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation 110 (2004): 2809-2816.
- Shepherd J, Cobbe SM, Ford I, et al. West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 333 (1995): 1301- 1307.
- Marchesini G, Bianchi G, Tomassetti S, et al. Metformin in non-alcoholic steatohepatitis. Lancet 358 (2001): 893-894.
- Nair S, Diehl AM, Wiseman M, Farr GH, et al. Metformin in the treatment of non-alcoholic steatohepatitis: A pilot open label trial. Aliment. Pharmacol Ther 20 (2004): 23-28.
- Uygun A, Kadayifci A, Isik AT, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 19 (2004): 537-544.
- Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 100 (2005): 1082-1090.
- Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 42 (2005): 641-649.
- De Oliveira CP, Stefano JT, De Siqueira, et al. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non - alcoholic steatohepatitis. Hepatol Res 38 (2008): 159-
- Idilman R, Mizrak D, Corapcioglu D, et al. Clinical trial: Insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 28 (2008): 200?208.
- Nobili V, Marcellini M, Devito R, et al. NAFLD in children: A prospective clinical-pathological study and effect of lifestyle advice. Hepatology 44 (2006): 458-465.
- Shields WW, Thompson KE, Grice GA, et al. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Ther Adv Gastroenterol 2 (2009): 157-163.
- Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: Pilot study of metformin for the treatment of non- alcoholic steatohepatitis. Aliment Pharmacol Ther 29 (2009): 172-182.
- Haukeland JW, Konopski Z, Eggesbo HB, et al. Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scand J Gastroenterol 44 (2009): 853-860.
- Wang CH, Leung CH, Liu SC, et al. Safety and effectiveness of rosiglitazone in type 2 diabetes patients with nonalcoholic Fatty liver disease. J Formos Med Assoc 105 (2006): 743-752.
- Akyuz F, Demir K, Ozdil S, et al. The effects of rosiglitazone, metformin, and diet with exercise in nonalcoholic fatty liver disease. Dig Dis Sci 52 (2007): 2359-2367.
- Idilman R, Mizrak D, Corapcioglu D, et al. Clinical trial: Insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 28 (2008): 200?208.
- Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355 (2006): 2297-2307.
- Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135 (2008): 1176-1184.
- Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: One-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 135 (2008): 100-110.
- Omer Z, Cetinkalp S, Akyildiz M, et al. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 22 (2010): 18-23.
- Tushuizen ME, Bunck MC, Pouwels PJ, et al. Incretin mimetics as a novel therapeutic option for hepatic steatosis. Liver Int 26 (2006): 1015-1017.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 24 (2008): 275-286.
- Kenny PR, Brady DE, Torres DM, et al. Exenatide in the treatment of diabetic patients with non-alcoholic steatohepatitis: A case series. Am J Gastroenterol 105 (2010): 2707-2709.
- Iwasaki T, Yoneda M, Inamori M, et al. Sitagliptin as a novel treatment agent for non-alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology 58 (2011): 2103-2105.
- Itou M, Kawaguchi T, Taniguchi E, et al. Dipeptidyl peptidase IV inhibitor improves insulin resistance and steatosis in a refractory nonalcoholic fatty liver disease patient: A case report. Case Rep Gastroenterol 6 (2012): 538-544.
- Yilmaz Y,Yonal O, Deyneli O, et al. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg 75 (2012): 240-244.
- Armstrong MJ, Houlihan DD, Rowe IA, et al. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: Individual patient data meta -analysis of the LEAD program. Aliment Pharmacol Ther 37 (2013): 234-242.
- Nelson A, Torres DM, Morgan AE, et al. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J Clin Gastroenterol 43 (2009): 990-994.
- Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: A post-hoc analysis. Lancet 376 (2010): 1916-1922.
- Foster T, Budoff MJ, Saab S et al. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: The St Francis Heart Study randomized clinical trial. Am J Gastroenterol 106 (2011): 71-77.
- Pramfalk C, Parini P, Gustafsson U, et al. Effects of high-dose statin on the human hepatic expression of genes involved in carbohydrate and triglyceride metabolism. J Intern Med 269 (2011): 333-339.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency inmeta-analyses. BMJ 327 (2003): 557-560.
- Shepherd J. TheWest of Scotland Coronary Prevention Study: A trial of cholesterol reduction in Scottish men. Am J Cardiol 76 (1995): 113C-117C.
- Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359 (2008): 2195-2207.
Citation: Jiang Hong, Hassah Batool Iftikhar. Primary Prevention of Cardiovascular Disease with Collaborative Diabetes Study in the Efficacy of Statins: Meta-Analysis of Randomized Controlled Trials. Cardiology and Cardiovascular Medicine 2 (2018): 123-134.
