Patients with Normal Coronary Arteries Via Angiography: The Relationship Between Slow Coronary Flow and Serglycin
Article Information
Hasan Ata Bolayir*
Department Cardiology, Sivas Numune Hospital; Sivas-Turkey
*Corresponding Author: Hasan Ata Bolayir, Department Cardiology, Sivas Numune Hospital; Sivas-Turkey
Received: 08 May 2018; Accepted: 30 May 2018; Published: 08 June 2018
Citation: Hasan Ata Bolayir. Patients with Normal Coronary Arteries Via Angiography: The Relationship Be tween Slow Coronary Flow and Serglycin. Cardiology and Cardiovascular Medicine 2 (2018): 111-122.
View / Download Pdf Share at FacebookAbstract
Background: Slow coronary flow (SCF) is an angiographic finding characterized with delayed opacification of epicardial coronary arteries without obstructive coronary disease. Several mechanisms have been proposed for SCF phenomenon, including inflammation. Serglycin has an important role in the inflammatory status. In this study, we aimed to investigate the relationship between serglycin and SCF phenomenon in patients with angiographically normal coronary arteries.
Method: A total of 174 individuals [n=92 with SCF and n=82 with NCF (normal coronary flow)] who underwent coronary angiography with suspicion of coronary artery disease, and had angiographically normal coronary arteries of varying coronary flow rates without any atherosclerotic lesion were enrolled. SCF was defined according to TIMI frame count (TFC) method.
Results: Baseline demographic properties were similar in both groups. Those with SCF had significantly increased average serum high sensitivity C-reactive protein (hsCRP), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), vascular cell adhesion protein-1 (VCAM-1) and serglycin values than those of NCF (hsCRP: 2.4 ± 0.4 vs. 1.1 ± 0.2mg/dL, p=0.006, TNF-α :5.95 ± 0.9 vs. 3.17 ± 0.6 pg/mL, p<0.001, IL-6: 22.97 ± 1.9 vs. 14.23 ± 1.1 pg/mL, p=0.002,VCAM-1:21.32 ± 3.9 vs. 10.1 ± 2.2 pg/mL, p<0.001, serglycin: 21.1 ± 2.9 vs. 12.3 ± 3.1 ng/mL, p<0.001, respectively).The ROC curve analysis showed a moderate diagnostic value of plasma serglycin levels for identifying patients with SCF from those with NCF (AUC = 0.804, 95% CI: 0.735–0.872, P < 0.001), it was better than that of plasma hsCRP levels (AUC = 0.617, 95% CI: 0.533–0.702, P = 0.010) and similar with that of plasma TNF-α levels (AUC = 0.785, 95% CI: 0.718–0.853, P < 0.001).
C
Keywords
Slow coronary flow; Coronary arteries; Coronary vasculature; Pericardium
Article Details
1. Introduction
Slow coronary flow (SCF) is an angiographic phenomenon that is defined by the slowed opacification of the coronary arteries located in the inner layer of the pericardium in the absence of coronary obstructive disease [1]. SCF is a somewhat prevalent angiographic result, as it is found in approximately 1% of patients who undergo coronary angiography for suspected coronary artery disease (CAD) [2]. It was first described in 1972 [1]; since then, there have only been a small number of publications regarding SCF, and because of this, the clinical implications and exact pathophysiological processes of this phenomenon have not yet been completely elucidated. However, there have been many proposed mechanisms, not limited to endothelial dysfunction, small vessel disease, diffuse atherosclerosis, microvascular vasomotor dysfunction, and inflammation [3-6]. It has also been suggested that a type of acute atherosclerosis, occlusive disease of the small coronary arteries, may also lead to the development of SCF [7]. Indeed, SCF patients with normal coronary arteries who also have angina may be at increased risk for transient myocardial hypoperfusion; further, this population has a poorer prognosis and a greater risk of significant coronary artery disease [8]. Serglycin is a protein that is typically associated with intracellular proteoglycan and hematopoietic cells; however, previous studies have reported that some non-hematopoietic cell types also synthesize serglycin. Serglycin is synthesized by inflammatory cells, which store the protein in granules until it is necessary to react with mediators, including cytokines, chemokines, growth factors, and proteases [9, 10]. Serglycin is known to participate in the mechanism of atheromatous change and atherosclerosis. It has been shown that serglycin levels can be increased by several different factors, including by macrophage-related lipopolysaccharides, endothelial cell-related tumor necrosis factor-alpha (TNF-α), and smooth muscle cell-related interleukin 1-beta (IL-1ß) [11]. These findings indicate that serglycin plays an important role in inflammation as well as in the progression of vascular diseases; therefore, we hypothesize that serglycin might play a role in the development of SCF. To our knowledge, the current study is the first of its kind to be published in the literature.
2. Methods
2.1 Design of the study
The current study was of an observational and cross-sectional nature.
2.1.1 Patient population: The current study included a total of 174 participants [n=92 SCF and n=82 controls (NCF, normal coronary flow)]. All of the included individuals had undergone outpatient coronary angiography due to suspected CAD (5/2015-3/2017) at our facilities. All of the study participants had normal coronary arteries (via angiography) with differing coronary flow rates, and none of them had atherosclerotic lesions. Every patient had experienced chest pain or symptoms indicative of angina via a study of his/her myocardial perfusion or a treadmill test. We confirmed that all of the participants conformed to the requirements of having cardiac syndrome-X, which were as follows: (1) having experiences of ang?na resulting almost solely on effort, and adequately normal to be indicative of CAD; (2) in instances of randomly occurring or elicited angina, having results indicative of anomalies in coronary blood flow or having myocardial ischemia; (3) having angiographically normal coronary arteries; (4) not showing signs of any other definitive cardiac diseases. In one visit to our clinic, every patient underwent a physical examination by a cardiology physician, his/her medical history was recorded, and he/she underwent cardiac catheterization. These data were collected and filed in our hospital’s coronary angiography laboratory. Exclusion criteria for this study included having pre-existing diseases (i.e., peripheral vascular or coronary), coronary arteries that were ectatic, having dilated cardiomyopathy that was not ischemic, dysfunctions in the renal or hepatic systems, having signs of continuing inflammation or infection, having dysfunctions in the hematological system, and having any malignancies. In addition, it should be noted that none of the individuals included in this study were taking any sort of vasoactive prescription drugs.
This study was conducted within the Declaration of Helsinki guidelines. Before the study began, all participants provided informed agreement.
2.1.2 Evaluating biochemical parameters:Blood was collected from each patient following a fasting period of 12 hours. Serum was separated via centrifugation at 4000 × g for 10 minutes, then it was frozen (-80°C) until further use. Blood collected from each patient at admission was analyzed for complete blood count and differentials. An automatized analyzer was used to measure high sensitivity C-reactive protein (hs-CRP), total cholesterol, triglycerides, creatinine, low density lipoprotein (LDL) cholesterol and high density lipoprotein (HDL) cholesterol. Serglycin, TNF-α, vascular cell adhesion protein-1 (VCAM-1) and interleukin-6 (IL-6) levels in plasma were evaluated via ELISA [8].
2.1.3 Angiographic evaluation of the coronary arteries and SCF diagnosis:The Judkins method was followed when performing coronary angiography, which was accomplished via the femoral approach. All of the study participants were given iopromide (Ultravist 370, Schering AG, Berlin, Germany) as the contrast medium. All participants underwent assessment for SCF via coronary angiography; the TFC was used to quantitate each patient’s coronary flow rate [12]. To be sure that the coronary flow was objectively quantitated, two separate clinicians who were unaware of the patient’s clinical information determined the coronary flow via TFC. With TFC, the operator must note the quantity of cine frames (taken at thirty frames per second) required so that the contrast is at the level of the normal distal coronary landmarks at the following arteries: left circumflex (LCX), left anterior descending (LAD), and right coronary arteries (RCA). Predefined distal landmarks are the distal bifurcation for the LAD, commonly referred to as the ‘pitchfork’or ‘whale’s tail’, the distal bifurcation of the segment with the longest total distance for the LCX, and the first branch of the posterolateral artery for the RCA. The accepted averages for normal coronary arteries are 22.2 ± 4.1 frames for LCX, 36.2 ± 2.6 frames for LAD, and 20.4 ± 3 frames for RCA. The TFC for the LAD is often the highest of the coronary arteries because it is the longest; because of this, the TFC for the LAD must be corrected by dividing by 1.7.
Following correction, the corrected TFC (cTFC) for the LAD is 21.1 ± 1.5 frames. For the current study, we considered the participants to be diagnosed with SCF if they had a cTFC that was more than 2 standard deviations from the reported range for each vessel. The average TFC for each study participant was determined by adding together the TFCs for the LCX, LAD, and RCA, and then dividing this number by 3. Vessel lengths and ostial diameters of the three coronary arteries were measured using quantitative coronary angiography (QCA).
2.1.4 Statistics:All data were evaluated using SPSS software (version 20.0 for Windows, Inc., Chicago, Illinois, United States of America). Variables that were continuous are presented as means and standard deviations, while variables that were categorical are presented as percentages, and were analyzed via a Chi-square test. A Kolmogorov-Smirnov test was used to determine whether data were normally distributed. Variables that were continuous, numerical, and normally distributed underwent a univariate analysis with two sample T test, while those that were not normally distributed were analyzed with a Mann-Whitney U test.
Logistic regression analysis was used to determine the independent risk factors for SCF. All significance tests were two-tailed, and values of p<0.05 were considered to be significant.
3. Results
3.1 Clinical characteristics
All of the study participants’ important clinical parameters are listed in Table 1. There were no significant differences between the SCF and control group regarding gender, age, presence of hypertension, history of smoking, and presence of diabetes (p>0.05). In addition, there was no difference in the lipid parameters or glucose values (following fasting) of the two groups (p>0.05).
|
Variables |
SCF (n=92) |
NCF (n=82) |
*p |
|
Age, years |
54±12 |
53±11 |
0.82 |
|
Male gender, n |
50 (%54) |
44 (%53) |
0.86 |
|
BMI, kg/m2 |
31±8 |
29±9 |
0.74 |
|
Hypertension, n |
31 (%33) |
27 (%32) |
0.88 |
|
Diabetes mellitus, n |
37 (%40) |
32 (%39) |
0.84 |
|
Hyperlipidemia, n |
22 (%24) |
18 (%22) |
0.64 |
|
Cigarette smoking, n |
28 (%30) |
24 (%29) |
0.84 |
|
Family history of CAD, n |
17 (%18) |
15 (%17) |
0.88 |
|
Fasting glucose, mg/dL |
112±46 |
100±23 |
0.62 |
|
Total cholesterol, mg/dL |
189±36 |
190±26 |
0.82 |
|
Triglycerides, mg/dL |
148±94 |
125±62 |
0.44 |
|
HDL cholesterol, mg/dL |
42±11 |
44±10 |
0.84 |
|
LDL cholesterol, mg/dL |
117±31 |
120±25 |
0.78 |
|
hsCRP, mg/dL |
2.4±0.4 |
1.1±0.2 |
0.006 |
|
TNF-α, pg/mL |
5.95±0.9 |
3.17±0.6 |
<0.001 |
|
IL-6, pg/mL |
22.97±1.9 |
14.23±1.1 |
0.002 |
|
VCAM-1, pg/mL |
21.32±3.9 |
10.1±2.2 |
<0.001 |
|
Serglycin,ng/mL |
21.1±2.9 |
12.3±3.1 |
<0.001 |
|
TIMI frame count measurements |
|||
|
LAD |
60±28 |
29±7 |
<0.001 |
|
LAD(corrected) |
35±17 |
17±4 |
<0.001 |
|
LCx |
29±11 |
20±3 |
<0.001 |
|
RCA |
41±23 |
19±5 |
<0.001 |
|
Mean |
35±12 |
19±3 |
<0.001 |
|
The length of epicardial coronary arteries |
|||
|
LAD,mm |
170±20 |
170±19 |
0.88 |
|
LCx,mm |
127±30 |
124±29 |
0.72 |
|
RCA,mm |
183±39 |
166±32 |
0.56 |
|
Diameters of coronary arteries |
|||
|
LAD,mm |
3.96±0.66 |
3.60±0.53 |
0.026 |
|
LCx,mm |
3.66±0.65 |
3.29±0.74 |
0.044 |
|
RCA,mm |
3.71±0.81 |
3.10±0.68 |
0.003 |
|
Coronary flow velocities |
|||
|
LAD,mm/s |
102.8±47.0 |
186.6±57.0 |
<0.001 |
|
LCx,mm/s |
148.7±59.6 |
203.7±61.5 |
0.001 |
|
RCA,mm/s |
165.9±73.9 |
280.1±76.8 |
<0.001 |
Data are presented as mean±SD, median (inter quartile range) and as number (per-centage).*Student’st-test, Mann-Whitney U test and Chi-square test; BMI- body mass index, HDL-high density lipoprotein, LAD-left anterior descending artery, LCx-left circumflex artery, LDL-low-density lipoprotein, NCF-normal coronary flow, NS-not significant, RCA-right coronary artery, SCF-slow coronary flow, hsCRP: high sensitivity C-reactive protein, TNF-α: tumor necrosis factor-alpha, IL-6: interleukin-6, VCAM-1: vascular cell adhesion protein-1.
Table 1: Demographic and clinical characteristics of study participants.
3.2 TFC
Not surprisingly, SCF patients had significantly increased TFC in all three of the major coronary arteries over those of the controls (p<0.05). While there were no differences in the lengths of the vessels amongst the SCF and control groups; the SCF patients had coronary arteries that were significantly greater in size than those of the control group, while the control group had significantly increased coronary flow rates over those of the SCF group (p<0.05). We did find that serglycin had a significant and positive correlation with average TFC [for all participants: r = 0.522, p < 0.001; for SCF patients: r = 0.568, p < 0.001; for normal controls: r = -0.292, p > 0.05] (Figure 1). In addition, hsCRP also had a significant and positive relationship with average TFC [for all participants: r = 0.511, p < 0.001; for SCF patients: r = 0.571, p < 0.001; for normal controls: r = -0.157, p >0.05].
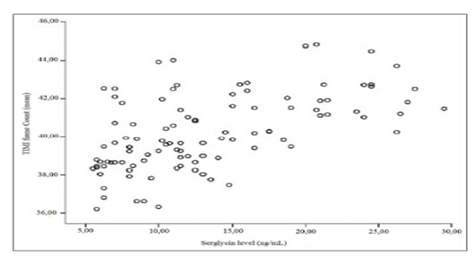
Figure 1: The correlation of serglcine levels with TIMI frame count in patients with SCF.
3.3 Correlations between serglycin and the other inflammatory markers with SCF
Those with SCF had significantly increased average serum hsCRP, TNF-α, IL-6, VCAM-1 and serglycin values than those of the controls (hsCRP: 2.4 ± 0.4vs.1.1 ± 0.2mg/dL, p=0.006, TNF-α:5.95 ± 0.9 vs. 3.17 ± 0.6 pg/mL, p<0.001, IL-6: 22.97 ± 1.9vs.14.23 ± 1.1 pg/mL, p=0.002,VCAM-1:21.32 ± 3.9vs.10.1 ± 2.2 pg/mL, p<0.001, serglycin:21.1 ± 2.9 vs. 12.3 ± 3.1 ng/mL, p<0.001, respectively). We found that average coronary diameter solidly estimated SCF (OR: 7.358, 95% CI: 1.990-27.20, p=0.003), while serglycin weakly estimated SCF (OR: 1.044, 95% CI: 1.006-1.084, p=0.023) via logistic regression analysis (Table 2). On the other hand there was an independent relationship between the other inflammatory markers with SCF (Table 3). We used a receiver operating characteristic (ROC) curve to determine the sensitivity and specificity of serglycin, TNF-α and hsCRP for the detection of SCF in those included in the study. Results (Figure 2) indicate that plasma serglycin levels had a significant predictive value for the identification of individuals with SCF versus those with NCF (AUC = 0.804, 95% CI: 0.735–0.872, P < 0.001), which was more determinative than that of plasma hsCRP (AUC = 0.617, 95% CI: 0.533–0.702, P = 0.010).
Further, our data suggest that 16.95 ng/mL is an appropriate cutoff value for plasma serglycin that could be used to separate those having SCF from those with NCF; this had a specificity of 70.2% and a sensitivity of 91.3%.
|
Variables |
Slow coronary flow (Dependent variable) |
|
|
OR ( 95%CI) |
*p |
|
|
Serglycin, ng/mL |
1.044 (1.006-1.084) |
0.023 |
|
Mean coronary diameter |
7.358 (1.990-27.20) |
0.003 |
Table 2: The independent relationship of Serglycin with slow coronary flow phenomenon.
|
Inflammatory marker |
OR (95% CI) |
p |
|
Hs-CRP |
1.55 (1.00–2.39) |
0.038 |
|
TNF-α |
2.78 (1.04–7.45) |
0.028 |
|
IL-6 |
1.73 (1.01–3.34) |
0.042 |
|
VCAM-1 |
2.13 (1.47–11.20) |
0.030 |
hsCRP: high sensitivity C-reactive protein, TNF-α: tumor necrosis factor-alpha, IL-6: interleukin-6, VCAM-1: vascular cell adhesion protein-1.
Table 3: The independent relationship of the other inflammatory markers with slow coronary flow phenomenon.
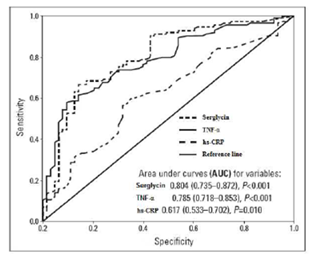
Figure 2: ROC curve analyses of the predictive power of plasma serglycin, TNF-α and hs-CRP levels.
4. Discussion
Herein, we show that SCF patients have significantly elevated serglycin values compared to those with NCF. Levels of inflammatory markers (TNF-α, VCAM-1, IL-6 and hs-CRP) were higher in subjects with SCF than those with NCF. We showed that there was a strong relation between systemic inflammation and SCF. As far as we know, the current study is the only study to show a correlation between SCF and serglycin.
SCF is an atherosclerotic condition that is mediated by inflammation. Several cells that are inflammatory and other types of mediators are known to heighten and maintain signals that are proinflammatory, which can lead to the onset and progression of SCF. It is known that many heart conditions are influenced by inflammation; in addition, inflammation is associated with many various clinical conditions of CAD. There has been a recent increase in the realization that inflammation that is chronic may play an important role in diseases of the cardiovascular system [13, 14]. In addition, there is an evidence that the activation of endothelial cells as well as inflammation may also imitate and sustain atherosclerotic diseases [15-17]. Recently, it has been proposed that inflammation plays a role in SCF. One study [18] found that serum VCAM-1, intercellular adhesion molecule-1 (ICAM-1), and E-selectin were significantly elevated in patients with SCF over those with coronary flow that was normal. Another study [19] revealed that SCF patients had heightened levels of inflammatory markers such as IL-6 and hsCRP. Two other studies showed that serum uric acid levels and red cell distribution width also had a relationship with SCF [20, 21].
Serglycin is an important proteoglycan that is secreted within immune cells; in these cells, serglycin interacts with several inflammatory-mediating molecules, including growth factors, proteases, cytokines, and chemokines [9, 10]. It has also been reported that circulating serglycin may play a role in the processes of atheromatous change and systemic vascular insult [22, 23]. Serglycin biosynthesis increases the amount of lipopolysaccharide in macrophages [22, 23], IL-1ß in smooth muscle cells, and TNF-α in endothelial cells [11].
Recently, it was reported that serglycin is one of the proteins with the highest expression levels in the epicardial adipose tissue of CAD patients. That paper also revealed that serglycin secretion in adipocytes is induced by TNF-α, suggesting that the interaction between TNF-α and serglycin may cause the onset and advancement of CAD via adipocyte/macrophage cross-talk [24]. They also presented here that serglycin expression in epicardial adipose tissue (EAT) is higher than that in subcutaneus adipose tissue, and that serglycin is expressed in adipocytes. EAT has been demonstrated to be an abundant source of pro-in?ammatory cytokines, such as TNF-α and IL-6 [25]. Substantial in?ammatory cell in?ltrates, predominantly represented by macrophages, have been observed in EAT obtained during cardiac surgery of subjects with severe CAD [26]. Considering that TNF-α is primarily produced by macrophages and monocytes [27], in?ammatory cells in EAT are likely the predominant source of TNF-α. In macrophages, serglycin has been shown to regulate secretion of TNF-α [28]. Tsubakimoto et al [24] demonstrated that TNF-α induces expression and secretion of serglycin in adipocytes. Therefore, serglycin and TNF-α probably regulate each other’s expression and secretion and mediate paracrine cross-talk between macrophages and adipocytes in EAT, which creates a vicious cycle of in?ammatory changes. Furthermore, TNF-α exerts potent pro-in?ammatory effects in atherosclerosis [27]. TNF-α also functions as a determinant of adipocytokine dysregulation in adipocytes of obese subjects [29]. Adipocytokines from EAT have been suggested to promote atheromatous plaque formation in the intima layer by passing into the myocardium via the vasa vasorum [30]. These observations suggest that serglycin and TNF-α in EAT likely contribute to the development and progression of CAD through crosstalk between macrophages and adipocytes. Although it remains unclear what tissue and cell type account for circulating serglycin, it is possible that circulating serglycin, incooperation with pro-in?ammatory proteins secreted from adipose tissue-derived macrophages, might be systemically involved in in?ammation in adipose tissues. TNF-α and IL-6 are important mediators of inflammation and could provide a potential link between visceral fat and systemic inflammation [31]. They are both known to promote lipolysis and the secretion of free fatty acids, which contribute to an increase in hepatic glucose output and insulin resistance, impair adipocyte differentiation, and promote inflammation. Both factors are released from the vessel wall during an inflammatory response and in turn stimulate the release of acute phase reactant hsCRP, which induces the expression of VCAM-1 [32]. VCAM-1 is believed to best reflect a proatherogenic state [33] In addition, serum levels of both VCAM-1, TNF-α and IL-6 are raised in patients with CAD [34].
Serglycin, TNF-α, VCAM-1 and IL-6 levels were higher in subjects with SCF than those with NCF in our study population. On the other hand, in the current study, we found that TFC was correlated with hsCRP; this may be because hsCRP is a marker of inflammation for the development of atherosclerosis and the associated patterns of coronary flow [35]. The role of CRP in microvessels that are angiogenic from tissues with disease has been elucidated and published [36]. It has been hypothesized that SCF is due to these abnormalities in the microvasculature [37]; in addition, IL-6 and CRP values were upregulated in patients with SCF. On the other hand, another study [35] reported that CRP and TFC were not significantly related to SCF. This is in contrast to the results of the current study, in which we found that hsCRP was significantly correlated to SCF. Based on all of the above studies, including the current study, we believe that dysregulations in inflammation may indicate dysfunction in the endothelial cells, which may lead to SCF. Because of this, we hypothesized that increases in SCF patient serglycin levels may be due to the fact that those with the disease suffer from ongoing inflammation. Our hypothesis was confirmed, as we found a significant and positive relationship amongst plasma serglycin and other inflammatory markers. An extensive analysis of the ROC curve also indicated that serglycin levels can independently estimate SCF. Further, we show that hsCRP, TNF-α and serglycin have comparable diagnostic values for SCF.
However, it should be noted that serglycin and TNF-α were stronger indicators of SCF than was hsCRP. Since atherosclerosis has been recognized as a chronic in?ammatory disease of the arterial wall [38-40], circulating serglycin and mediators of in?ammation might also participate in the mechanisms of systemic vascular insult and atheromatous change. Further, another study [41] reported that serglycin levels in coronary artery ectasia (CAE) patients were significantly increased over those of controls. CAE is considered to be a large positive remodeling of the atherosclerotic coronary artery [42]. The most emphasized mechanisms of this remodeling are the enzymatic degradation of the extracellular matrix and the thinning of the tunica media layer of the vessel due to severe chronic inflammation [43]. According to Kundi et al. [41] serglycin level is significantly and independently higher in patients with CAE. Their findings are supporting that inflammation may in part have a role for the development of CAE.
Similarly we found that there was a strong correlation between SCF and mean coronary diamater in our study population. In conclusion, the current study revealed that SCF patients have significantly increased plasma serglycin levels than those with NCF. In addition, we also found that plasma serglycin is an independent risk factor of SCF.
5. Limitations of this Study
While this study brings valuable insight to the field, it does have some limitations. One limitation is that we enrolled a small number of participants. If we had enrolled a larger number of participants, we would have had more statistical power. Next, which may be the most important limitation, is that this study was observational. Therefore, we were unable to determine a clear method to explain the correlation among SCF and the upregulation of serglycin. In addition, our study participants were not subjected to IVUS for the detection of atherosclerotic variations in their coronary arteries. Because of this, we could not exactly be sure whether patients with ‘isolated’ SCF also had non-obstructive CAD. However, patients with SCF are not typically subjected to IVUS; they often receive a diagnosis of SCF via angiographic methods. Finally, the participants used as controls were not totally normal. While they had coronary arteries that were normal via angiography, all of them had some risk factors for cardiac disease or had cardiac syndrome- X. Because of this, we may have missed some significant differences between the two groups. However, it is important to note that in spite of all of the limitations, this study led to many significant findings regarding serglycin and SCF.
References
- Tambe AA, Demany MA, Zimmerman HA, et al. Angina pectoris and slow flow velocity of dye in coronary arteries--a new angiographic finding. Am Heart J 84 (1972): 66-71.
- Goel PK, Gupta SK, Agarwal, et al. Slow coronary flow: a distinct angiographic subgroup in syndrome X. Angiology 52 (2001): 507-514.
- Bolayir A, Cigdem B, Gokce SF, et al. The Effect of Eosinopenia on Mortality in Patients with Intracerebral Hemorrhage. Journal of Stroke and Cerebrovascular Disease 10 (2017): 2248-2255.
- Bolayir A, Gokce SF, Cigdem B, et al. Monocytes/high-density lipoprotein ratio predicts the mortality in ischemic stroke. Neurol Neurochir Pol (2017).
- Sezgin AT, S???rc? A, Barutcu I, et al. Vascular endothelial function in patients with slow coronary flow. Coron Artery Dis 14 (2003): 155-161.
- R?za Erbay A, Turhan H, Ya?ar AS, et al. Elevated level of plasma homocysteine in patients with slow coronary flow. Int J Cardiol 102 (2005): 419-423.
- Mosseri M, Yarom R, Gotsman MS, et al. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation 74 (1986): 964-972.
- Fragasso G, Chierchia SL, Arioli F, et al. Coronary slow-flow causing transient myocardial hypoperfusion in patients with cardiac syndrome X: long-term clinical and functional prognosis. Int J Cardiol 137 (2009): 137-144.
- Kolset SO, Pejler G. Serglycin a structural and functional chameleon with wide impact on immune cells. The journal of Immunology 187 (2011): 4927-4933.
- Niemann CU, Abrink M, Pejler G, et al. Neutrophil elastase depends on serglycin proteoglycan for localization in granules. Blood 109 (2007): 4478-4486.
- Korpetinou A, Skandalis SS, Labropoulou VT, et al. Serglycin: at the crossroad of inflammation and malignancy. Frontiers in oncology (2013).
- Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 93 (1996): 879-888.
- Sullivan GW, Sarembock IJ, Linden J. The role of inflammation in vascular disease. J Leukoc Biol 67 (2000): 591-602.
- Gokce M, Kaplan S, Tekelio?lu Y, et al. Platelet function disorder in patients with coronary slow flow. Clin Cardiol 28 (2005): 145-148.
- Camsar? A, Pekdemir H, Cicek D, et al. Endothelin-1 and nitric oxide concentrations and their response to exercise in patients with slow coronary flow. Circ J 67 (2003): 1022-1028.
- Y?ld?z A, Gur M, Y?lmaz R, et al. Association of paraoxonase activity and coronary blood flow. Atherosclerosis 197 (2008): 257-263.
- Enli Y, Turk M, Akbay R, et al. Oxidative stres parameters in patients with slow coronary flow. Adv Ther 25 (2008): 37-44.
- Turhan H, Saydam GS, Erbay AR, et al. Increased plasma soluble adhesion molecules; ICAM-1, VCAM-1, and E-selectin levels in patients with slow coronary flow. Int J Cardiol 108 (2006): 224-230.
- Li JJ, Xu B, Li ZC, et al. Is slow coronary flow associated with inflammation? Med Hypotheses 66 (2006): 504-508.
- Kalay N, Aytekin M, Kaya MG, et al. The relationship between inflammation and slow coronary flow: increased red cell distribution width and serum uric acid levels. Turk Kardiyol Dern Ars 39 (2011): 463-468.
- Yildiz A, Yilmaz R, Demirbag R, et al. Association of serum uric acid level and coronary blood flow. Coron Artery Dis 18 (2007): 607-613.
- Zernichow L, Åbrink M, Hallgren J, et al. Serglycin is the major secreted proteoglycan in macrophages and has a role in the regulation of macrophage tumor necrosis factor-α secretion in response to lipopolysaccharide. Journal of biological chemistry 281 (2006): 26792-26801.
- Kolseth IBM, Reine TM, Vuong TT, et al. Serglycin is part of the secretory repertoire of LPS activated monocytes. Immunity, inflammation and disease 3 (2015): 23-31.
- Imoto-Tsubakimoto H, Takahashi T, Ueyama T, et al. Serglycin is a novel adipocytokine highly expressed in epicardial adipose tissue. Biochemical and biophysical research communications 432 (2013): 105-110.
- Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab 22 (2011): 450-457.
- Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of in?ammatory mediators. Circulation 108 (2003): 2460-2466.
- Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis (): a comprehensive review of studies in mice. Cardiovasc. Res 79 (2008): 360-376.
- Zernichow L, Abrink M, Hallgren J, et al. Serglycin is the major secreted proteoglycan in macrophages and has a role in the regulation of macrophage tumor necrosis factor-alpha secretion in response to lipopolysaccharide. J. Biol. Chem 281 (2006): 26792-26801.
- Maury E, Noel L, Detry R, et al. In vitro hyperresponsiveness to tumor necrosis factor-alpha contributes to adipokine dysregulation in omental adipocytes of obese subjects. J. Clin. Endocrinol. Metab 94 (2009): 1393-1400.
- Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am. J. Physiol. Endocrinol Metab 303 (2012): E937-E949.
- Koster A, Stenholm S, Alley DE, et al. Health ABC Study. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 18 (2010): 2354-2361.
- Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem 42 (2009): 1331-1346.
- Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis 170 (2003): 191-203.
- Corcoran TB, Engel A, Sakamoto H, et al. The effects of propofol on lipid peroxidation and in?ammatory response in elective coronary artery bypass grafting, J. Cardiothorac. Vasc. Anesth 18 (2004): 592-604.
- Pekdemir H, Cin VG, Çiçek D, et al. Slow coronary flow may be a sign of diffuse atherosclerosis (): contribution of FFR and IVUS. Acta Cardiol 59 (2004): 127-133.
- Slevin M, Krupinski J. A role of C-reactive protein in the regulation of angiogenesis, endothelial cell inflammation and thrombus formation in cardiovascular disease. Histol Histopathol 24 (2009): 1473-1478.
- Yaz?c? M, Aksakal E, Demircan S, et al. Is slow coronary flow related with inflammation and procoagulant state? Anadolu Kardiyol Derg 5 (2005): 3-7.
- Ross R. Atherosclerosis-an inflammatory disease. N. Engl. J. Med 340 (1999): 115-116.
- Stoll LL, Denning GM, Weintraub NL. Potentaik role of endotoxin as a proinflammatory mediator of atherosclerosis. Aterioscler. Thromb. Vasc. Biol 24 (2004): 2227-2236.
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options, Nat. Med 17 (2011): 1410-1422.
- Kundi H, Gok M, Topcuoglu C, et al. Association of Serglycin levels with isolated coronary artery ectasia. Kardiologia Polska (2017).
- Paik DC, Ramey WG, Dillon J, et al. The nitrite/elastin reaction: implication for in vivo degenerative effects. Connective tissue research 36 (1997): 241-251.
- Antoniadis AP, Chatzizisis YS, Giannoglou GD. Pathogenetic mechanisms of coronary ectasia. International journal of cardiology 130 (2008): 335-343.
Citation: Hasan Ata Bolayir. Patients with Normal Coronary Arteries Via Angiography: The Relationship Between Slow Coronary Flow and Serglycin. Cardiology and Cardiovascular Medicine 2 (2018): 156-167.
