Oviposition Behavior of Culex quinquefasciatus and Anopheles coluzzii Females According to the Ovitrap Color and Presence of Fertilizer in Breeding Sites
Article Information
Djiappi-Tchamen Borel1,2, Nchoutpouen Elysée3, Talipouo Abdou1,3, NKahe Diane Leslie1,3, Bamou Roland1,2, Kopya Edmond1,3, Awono-Ambene Parfait1, Tchuinkam Timoléon2, Christophe Antonio-Nkondjio1,4*
1Laboratoire de Recherche sur le Paludisme, Organisation de Coordination pour la lutte Contre les Endémies en Afrique Centrale (OCEAC), P.O. Box 288, Yaoundé, Cameroon
2Vector Borne Diseases Laboratory of the Applied Biology and Ecology Research Unit (VBID-URBEA), Department of Animal Biology, Faculty of Science, University of Dschang, P.O. Box 067, Dschang, Cameroon
3Department of Animal Physiology and Biology, Faculty of Science, University of Yaoundé I, P.O. Box 337, Yaoundé, Cameroon
4Vector Biology Liverpool School of Tropical medicine Pembroke Place, Liverpool, L3 5QA, UK
*Corresponding Author: Christophe Antonio-Nkondjio, Laboratoire de Recherche sur le Paludisme, Organisation de Coordination pour la lutte Contre les Endémies en Afrique Centrale (OCEAC), P.O. Box 288, Yaoundé, Cameroon; Vector Biology Liverpool School of Tropical medicine Pembroke Place, Liverpool, L3 5QA, UK
Received: 01 January 2021; Accepted: 13 January 2021; Published: 23 January 2021
Citation:
Djiappi-Tchamen Borel, Nchoutpouen Elysée, Talipouo Abdoul, NKahe Diane Leslie, Bamou Roland, Kopya Edmond, Awono-Ambene Parfait, Tchuinkam Timoléon, Christophe Antonio-Nkondjio. Oviposition Behavior of Culex quinquefasciatus and Anopheles coluzzii Females According to the Ovitrap Color and Presence of Fertilizer in Breeding Sites. Fortune Journal of Health Sciences 4 (2021): 207-220.
View / Download Pdf Share at FacebookAbstract
Malaria and lymphatic filariasis are still important public health problems in Cameroon and in most part of sub-Saharan Africa. However there is still not enough informations on the bionomic and oviposition behaviour of their respectives vectors. The present study assesses the influence of color and organics pollutants presence on Culex and Anopheles coluzzii females oviposition behaviour. Laboratory experiments using Culex quinquefasciatus and Anopheles coluzzii laboratory colonies were conducted. Gravid females were offered cups of different colors (red, green, yellow, black, purple and white) with water for oviposition. Experiments were also conducted with cups containing different concentration of NPK fertilizers (1%, 0.75%, 0.5% and 0%). The influence of NPK fertilizer on the larval development time was also assessed.
Cx quinquefasciatus and Anopheles coluzzii gravids females were found to be attracted by dark colors over 50% of eggs were laid in cups of black, red or green colors. The experiment was conducted with the mother and the F1 and F2 generations and similar oviposition preference were registered. When mosquitoes were offered cups containing different concentration of NPK for oviposition it appeared that females of the two species prefer laying their eggs in low concentration of NPK. Rearing of larvae in water containing NPK fertilizers was found to accelerate the larval development time of Culex quinquefasciaus but not of Anopheles coluzzii.
The study indicated that several factors could be influencing vector bionomic and need further understanding to improve the fight against vector populations.
Keywords
Anopheles coluzzii; Color; Culex quinquefasciatus; Experiments; NPK
Anopheles coluzzii articles; Color articles; Culex quinquefasciatus articles; Experiments articles; NPK articles, Anopheles coluzzii articles Anopheles coluzzii Research articles Anopheles coluzzii review articles Anopheles coluzzii PubMed articles Anopheles coluzzii PubMed Central articles Anopheles coluzzii 2023 articles Anopheles coluzzii 2024 articles Anopheles coluzzii Scopus articles Anopheles coluzzii impact factor journals Anopheles coluzzii Scopus journals Anopheles coluzzii PubMed journals Anopheles coluzzii medical journals Anopheles coluzzii free journals Anopheles coluzzii best journals Anopheles coluzzii top journals Anopheles coluzzii free medical journals Anopheles coluzzii famous journals Anopheles coluzzii Google Scholar indexed journals Color articles Color Research articles Color review articles Color PubMed articles Color PubMed Central articles Color 2023 articles Color 2024 articles Color Scopus articles Color impact factor journals Color Scopus journals Color PubMed journals Color medical journals Color free journals Color best journals Color top journals Color free medical journals Color famous journals Color Google Scholar indexed journals Culex quinquefasciatus articles Culex quinquefasciatus Research articles Culex quinquefasciatus review articles Culex quinquefasciatus PubMed articles Culex quinquefasciatus PubMed Central articles Culex quinquefasciatus 2023 articles Culex quinquefasciatus 2024 articles Culex quinquefasciatus Scopus articles Culex quinquefasciatus impact factor journals Culex quinquefasciatus Scopus journals Culex quinquefasciatus PubMed journals Culex quinquefasciatus medical journals Culex quinquefasciatus free journals Culex quinquefasciatus best journals Culex quinquefasciatus top journals Culex quinquefasciatus free medical journals Culex quinquefasciatus famous journals Culex quinquefasciatus Google Scholar indexed journals Experiments articles Experiments Research articles Experiments review articles Experiments PubMed articles Experiments PubMed Central articles Experiments 2023 articles Experiments 2024 articles Experiments Scopus articles Experiments impact factor journals Experiments Scopus journals Experiments PubMed journals Experiments medical journals Experiments free journals Experiments best journals Experiments top journals Experiments free medical journals Experiments famous journals Experiments Google Scholar indexed journals NPK articles NPK Research articles NPK review articles NPK PubMed articles NPK PubMed Central articles NPK 2023 articles NPK 2024 articles NPK Scopus articles NPK impact factor journals NPK Scopus journals NPK PubMed journals NPK medical journals NPK free journals NPK best journals NPK top journals NPK free medical journals NPK famous journals NPK Google Scholar indexed journals T2D articles T2D Research articles T2D review articles T2D PubMed articles T2D PubMed Central articles T2D 2023 articles T2D 2024 articles T2D Scopus articles T2D impact factor journals T2D Scopus journals T2D PubMed journals T2D medical journals T2D free journals T2D best journals T2D top journals T2D free medical journals T2D famous journals T2D Google Scholar indexed journals
Article Details
Introduction
Mosquitoes are largely distributed worldwide [1,2]. They are also vectors of several diseases to human and animals. Anopheline species such as members of the Anopheles gambiae complex are efficient vectors of malaria and filariasis [3,4]. Malaria is still an important public health threat in Cameroon with the whole country exposed to the risk of transmission [5]. Culex mosquitoes are responsible for high nuisance and the transmission of several diseases such as arboviruses and filariasis particularly in East Africa [6,7]. These species preferentially breed in permanent and/or semi-permanent, organically polluted water collections [8]. Studies conducted so far have reported high implication of Culex species in the transmission of lymphatic filariasis [9], avian malaria [10], and arboviruses such as West Nile virus [11]. In Cameroon, Lymphatic filariasis is considered to be endemic with mean prevalence level (ICT>1%) estimated at 3.3% countrywide [12]. Culex quinquefasciatus, one of the members of the Culex pipiens complex is largely distributed in urban settings of Cameroon [8,13]. Although their direct implication in LF transmission in Cameroon is still not well documented [8], previous studies indicated frequent circulation of arboviruses in Culex mosquitoes species [14,15].
To improve vector borne disease surveillance it is important to monitor the evolution of both Culex species and Anopheles gambiae s.l species as these are largely distributed across the country [3,8]. Main control measures used to control these vectors include the use of long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) [16,17]. These tools between 2000 and 2015, significantly contributed to the decrease of malaria morbidity and mortality across the world [17]. However, their efficiency is now affected by the rapid expansion of insecticide resistance and changes in the biting and resting behavior of mosquitoes [8,18].
In this context, understanding vector behavior, in particular oviposition behavior and tolerance to organic pollutants could provide key information that could be used to design new control strategies for better vector management. The information on vector population behavior could also be used for surveillance activities and to better appraise the impact of control interventions. Ovitraps have been widely used for monitoring mosquito populations and this sampling technique is considered as efficient, easy to use, cheap and sensitive [19]. In Cameroon, studies conducted so far in the city of Yaoundé indicated high adaptation of both anopheline and culex species to polluted habitats [20]. Further research on An. gambiae complex reported high tolerance of An. coluzzii to polluted habitats in urban area compare to An. gambiae s.s [21]. Yet so far there have not been many studies assessing the influence of pollution and color on anopheline or culex females choice of oviposition site. In urban areas mosquitoes are highly prevalent in agricultural cultivated sites because of the availability of standing water collections where fertilizers are commonly used by farmers [22] to enhance their productivity. These compounds are considered to alter the physical and chemical properties of aquatic habitats making them more suitable for mosquitoes. Many studies revealed that fertilizer used in rice fields are associated with a dramatic increase of mosquito larvae populations [23,24]. The influence of these compounds on vector bionomic and fitness of major vectors remain poorly understood.
In the present study, a series of laboratory experiments were conducted to assess the influence of color and presence of NPK fertilizer in breeding sites on the oviposition behavior of females Culex quinquefasciatus and An. coluzzii.
Materials and Methods
Establishment of laboratory colonies of Culex quinquefasciatus and Anopheles coluzzii
Immatures stages of Culex quinquefasciatus and Anopheles coluzzii mosquitoes were collected in eight districts of Yaoundé city using the deeping method [25] and were reared to the adult stage in OCEAC insectary under standard laboratory conditions (27-28ºC temperature; 70-80% hygrometry). Pupae were collected daily and transferred in cages (30 × 30 × 30 cm). Adult mosquitoes were provided continuous access of 10% glucose solution. For blood meal, Culex were fed overnight (7 pm) on chicken whereas Anopheles were fed on rabbit. Culex species collected were all identified as Culex quinquefasciatus using the morphological identification keys of Jupp. Anopheline collected were confirmed as Anopheles coluzzii after molecular identification [26,27]. The identified Culex quinquefasciatus and Anopheles coluzzii were used to establish laboratory colonies.
Influence of ovitrap’s color on the oviposition behavior of Culex quinquefasciatus and Anopheles coluzzii females
In order to find out whether Culex quinquefasciatus females during oviposition are attracted by certain colors, six colored (black, green, yellow, purple, red and transparent) plastics cups of 50 ml capacity containing spring water and two leaves of Bermuda grass as an oviposition attractant for gravids Culex mosquitoes [28] were placed in a cage. The cups were placed according to a clockwise direction and the transparent cup was used as control (Figure 1).
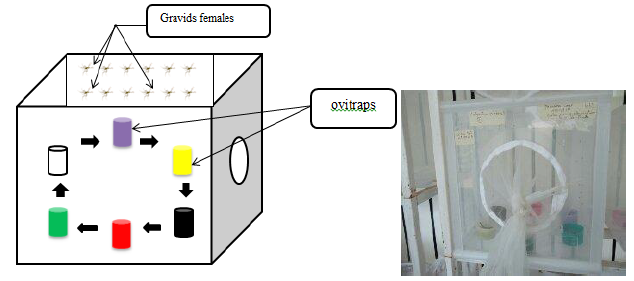
Figure 1: Experimental design of the study
One hundred of five to seven days old gravids females of Culex quinquefasciatus were introduced in cages to lay eggs. Every morning, the ovitraps were examined and eggs rafts laid in each cups were removed, counted and recorded. The dead females were removed and not replaced. To avoid bias, position of ovitraps was changed daily by circular rotation of cups. The experiment lasted 18 days for each mosquito generation with a variable number of replicates according to generation.
For experiments with Anopheles coluzzii only 35 gravid females were introduced per cage with seven colored cups (black, green, yellow, purple, red, white and transparent). The experiment was conducted similarly as the previous describe for Culex and was also performed with F0, F1 and F2 generations to check whether there was a change in the oviposition behavior from one generation to another.
NPK fertilizers influence on the oviposition behavior of Culex quinquefasciatus and Anopheles coluzzii
NPK used in this study is a fertilizer containing the following components nitrogen (N), phosphorus (P) and potassium (K) at similar proportion [29]. Four differents solutions of NPK at different concentrations (1%, 0.75%, 0.5% and 0%) were prepared. The 1% solution was obtained by adding 1mg of NPK in 100ml of distilled water. No NPK was added in the control cup. The cups were placed in a cage with Culex quinquefasciatus or An. coluzzii gravid females.
A total of 500 gravids Cx. quinquefasciatus and 175 gravids An. coluzzii females were distributed into 05 cages for eggs laying, 100 Culex and 35 anopheles females per cage were used. The ovitraps were rotated every two days to minimize a position bias. Dead females were removed and not replaced. The number of eggs laid in each cup were removed, counted using a stereomicroscope and recorded every morning.
Effect of the NPK fertilizers on the fitness of Culex quinquefasciatus and Anopheles coluzzii larvae
Eggs collected from each cup were reared in bigger containers. For each species, the first instar larvae (L1) obtained after eggs hatching were transferred in a container with a similar concentration of NPK as the cup with thirty five to sixty larvae/tray respectively. Larvae reared in clean water served as control. All larvae were fed with the same amount of food and were maintained under standard insectary conditions. Dead larvae were not replaced and the time to pupation was monitored. The experiment was replicated 12 times.
Data analysis
Oviposition activity index
The oviposition activity index (OAI) was determined according to the Kramer and Muller formula (1979):
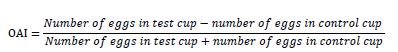
The index value varies from -1 to +1, with 0 indicating neutral response, Positives values are indicative of a positive attraction of the mosquito by a color compared to the control. A negative index is indicative of less attraction or deterrent/repellent effect of the oviposition cup.
The mean number of eggs per ovitrap and per concentration was calculated. A General Linear Model (GLM) using a correction of « quasipoisson » was used to evaluate the influence of the ovitrap’s color on the number of egg rafts and eggs laid by Culex and Anopheles coluzzii. The same analysis was carried out to evaluate the effect of fertilizer concentration on the above-mentioned parameters. A post-hoc Tukey-HSD (High Significance Difference) in the package « agricolae » [30] was used for pairwise comparisons. A GLM was also used to evaluate the influence of NPK fertilizers concentration on the duration of larval development of both species. Box-plots were drawn using the « ggplot » package. All the data were analyzed using R version 3.5.2 [31] and results with P < 0.05% was considered to be statistically significant.
Results
Influence of color on the oviposition behavior of Culex quinquefasciatus and Anopheles coluzzii gravids females
Out of the six color used for the experiment with Culex quinquefasciatus gravid females, it appeared that black color cups registered the highest eggs rafts compared to the others (F ratio = 10.264; P = 4.426e-07). The preference for dark colors particularly black color was similar for the different generations tested (Table 1). The average egg rafts in black color cups was 13.45 ± 10.33 eggs rafts while it was 1.90 ± 2.38 egg rafts for the purple which scored the lowest average egg rafts: (Table 1).
Table 1: Egg rafts of Culex quinquefasciatus distribution according to color during the F0, F1 and F2 generations
|
Cups colors |
||||||
|
Eggs rafts means ± CI 95% |
Black |
Green |
Red |
Transparent (Control) |
Yellow |
Purple |
|
Number of replicates (F0 generation) |
04 |
04 |
04 |
04 |
04 |
04 |
|
Eggs raft mean ± CI CI 95% |
21.60 ± 18.93 |
11.60 ± 10.16 |
9.40 ± 8.23 |
7.40 ± 6.48 |
6.80 ± 5.96 |
3.60 ± 3.15 |
|
F = 6.789; P = 0.0004491 |
||||||
|
Number of replicates (F1 generation) |
03 |
03 |
03 |
03 |
03 |
03 |
|
Eggs raft mean ± CI CI 95% |
4 ± 4.52 |
2.0 ± 2.26 |
3.33 ± 4.04 |
1.33 ± 1.50 |
0 ± 0 |
0.33 ± 0.37 |
|
F = 1.111; P = 0.2065 |
||||||
|
Number of replicates (F2 generation) |
03 |
03 |
03 |
03 |
03 |
03 |
|
Eggs raft mean ± CI CI 95% |
9.33 ± 10.55 |
1 ± 1.13 |
2 ± 2.26 |
0.66 ± 0.74 |
0± 0 |
0.66 ± 0.74 |
|
F = 11.72; P = 0.0002789 |
||||||
F = F-ratio; P = P value; (*) Data followed by different letters are significantly different at the 5% level; N = number of replications
The oviposition activities index (OAI) values were positive and high for black color cups while these values were negative for the purple and yellow color cups who display a repellent effect (Figure 2).
The black color cups were also prefer by ovipositing gravid Anopheles coluzzii females (Table 2). A significantly, high number of eggs were recorded in black color ovitraps. This trend did not significantly vary between the three different mosquito generations monitored in the course of the study (P=4.524e-13; F=18.5008) (Table 2). Green cups also appeared to be highly preferred by An. coluzzii females for oviposition (Table 2).
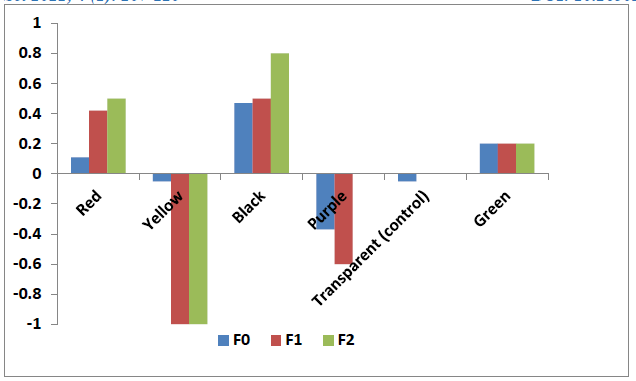
Figure 2: Oviposition activity index of Culex quinquefasciatus for different colors
Table 2: Oviposition sites color preferred by Anopheles coluzzii gravid females
|
Ovitraps’scolors |
Black |
Red |
Green |
Transparent (Control) |
White |
Yellow |
Purple |
||
|
Number of replicates (F0 Generation) |
04 |
04 |
04 |
04 |
04 |
04 |
04 |
||
|
Eggs means ± CI CI 95% |
156.50 ± 186.53 |
27 ± 44.20 |
102.25 ± 30.42 |
4.5 ± 8.81 |
10.5 ± 20.57 |
54.25 ± 72.99 |
3.25 ± 6.36 |
||
|
F = 2.104; P = 0.09606 |
|||||||||
|
Number of replicates (F1 Generation) |
04 |
04 |
04 |
04 |
04 |
04 |
04 |
||
|
Eggs means ± CI CI 95% |
258.25 ± 113.26 |
53 ± 21.73 |
59.50 ± 51.32 |
4.25 ± 8.32 |
0.25 ± 0.48 |
16.75 ± 32.82 |
3 ± 5.87 |
||
|
F = 13.37; P = 3.183e-06 |
|||||||||
|
Number of replicates (F2 Generation) |
04 |
04 |
04 |
04 |
04 |
04 |
04 |
||
|
Eggs means ± CI CI 95% |
243.75 ± 38.59 |
96.25 ± 59.73 |
105.25 ± 89.86 |
21.75 ± 14.84 |
25.50 ± 24.87 |
25.25 ± 24.87 |
31 ± 19.31 |
||
|
F = 11.8; P = 8.449e-06 |
|||||||||
F = F-ratio; P = P value; (*) Data followed by differents letters are significantly differents at the 5% level; N = number of replicates
Influence of NPK presence on the oviposition behavior of gravid females of Culex quinquefasciatus and Anopheles coluzzii
In laboratory, Cx. quinquefasciatus was found to prefer laying eggs in ovitraps with no NPK fertilizer. Control cups with no fertilizer recorded the highest number of egg rafts (15.60 ± 6.42). Cups with different NPK concentrations had a similar attraction to gravid females with egg rafts varying from 3.80 ± 2.77 for the 0.5% NPK to 4 ± 1 for the cup with 1% NPK (Figure 3).
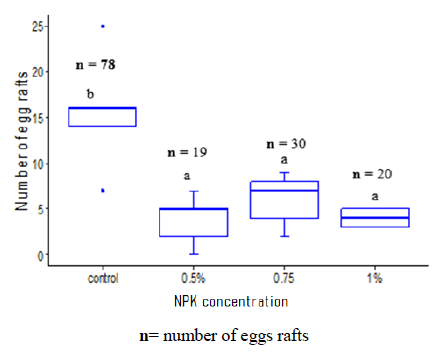
Figure 3: Eggs rafts Culex quinquefasciatus distribution according to different NPK concentrations
For gravids Anopheles coluzzii females it appeared that high density of eggs was recorded in the control cups and cups with a concentration of 0.75% of NPK (Figure 4).
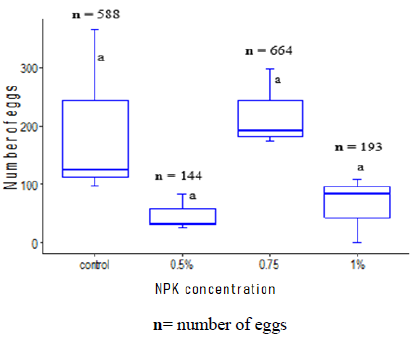
Figure 4: Number of eggs laid by Anopheles coluzzii females according to different concentrations of NPK fertilizers
Assessment of the NPK fertilizer influence on the larval development of Cx quinquefasciatus
Table 3 shows the influence of fertilizer on Culex larval development. Significant variations between larval development time and pupae productivity in relation with NPK concentrations was recorded (F= 3; p< 0.001). Out of 3120 Cx. quinquefasciatus (L1) larvae reared in water with NPK fertilizer, only 935 (29.96%) succeeded to the pupae stage whereas in the control group, out of 1200 (L1) larvae, 850 (70.83%) emerged as pupae. Larval development time was found to be shorter (about 8 days) with high concentration of NPK and longer in low concentration (12 days) and control group (10.60 days) (Table 3).
Table 3: Influence of NPK fertilizers on Culex quinquefasciatus larvae development
|
NPK Concentrations |
Number of exposed larvae |
N |
Development time from 1st instar to pupae (Min ± Max), Mean ± SD |
pupae productivity (%) |
|
0% (control) |
1200 |
20 |
(16-24) 10.60 ± 3.73a |
70.83% |
|
0.5% |
1200 |
20 |
(6-23) 12.14 ± 3.86b |
41.33% |
|
0.75% |
1200 |
20 |
(6-14) 8.36 ± 2.01c |
11.83% |
|
1% |
720 |
12 |
(6-13) 8.44 ± 1.99c |
41.25% |
MLD = Mean larval development; N = Number of replications; Min = Minimal value; Max = Maximal value; SD = Standard deviation
The number of pupae obtained was very high in control sample compare to the other groups out of 1200 first instar larvae, 850 successfully arrived at the pupae stage. Within the other groups (0.5%; 0.75%; 1%), small differences of pupae production was observed.
Effect of the presence of the chemical fertilizers (NPK) on larval development of Anopheles coluzzii larvae
Anopheles coluzzii larvae were found to survive well to different NPK fertilizers concentrations (F=3; P=0.09). Whereas there was no significant difference on larval development time according to various concentrations of NPK fertilizers (Table 4). The proportion of first instar larvae reaching the pupae stage was high in the control group compare to the remaining groups. Also high concentration of NPK were found to induce a high mortality with only 43.2% of larvae succeeding to the pupae stage with NPK 1% (Table 4).
Table 4: Effect of exposure to NPK fertilizers on Anopheles coluzzii larvae
|
NPK Concentrations |
Number of exposed larvae |
N |
Development time from 1st instar to pupae (Min-Max), Mean ± SD |
pupae productivity (%) |
|
0% (control) |
560 |
16 |
(16-24) 10.43 ± 1.58a |
80.17% |
|
0.5% |
280 |
08 |
(6-23) 10.36 ± 1.32a |
54.42% |
|
0.75% |
280 |
08 |
(6-14) 10.89 ± 1.2a |
48.21% |
|
1% |
175 |
05 |
(6-13) 10.88 ± 1.5a |
43.42% |
N = Number of replicates; Min = Minimal value; Max = Maximal value; SD = Standard deviation
Despite similar lives span among larvae within each group, the control sample appeared to produce more pupae than the others.
Discussion
The study objectives were to assess the oviposition behaviour of both Anopheles coluzzii and Culex quinquefasciatus females. The study suggested that Culex quinquefasciatus and Anopheles coluzzii gravid females prefer laying eggs in black or dark colour cups. The attractiveness of Culex females by dark colour could be explain by the fact that the black color mimic shading water body such as dark gutter or pit latrine were Culex quinquefasciatus larvae are usually found [8]. Moreover, black containers are also known to absorb light across most of the visible spectrum than other colors and might reduce the exposition of larvae to sun shine effects [32,33]. Darkness and wetness were also identified as critical positive cues for Anopheles gambiae oviposition [32-34]. Females anopheline were also found to oviposit in different cups at a given time. This behavior also known as skip oviposition have been reported elsewhere [34] and could be part of an oviposition strategy which has as objective to disperse egg laid in order to give more chances to the progeny to grow to the adult stage. Many mosquitoes were reported to choose their oviposition site on the basis of the physico-chemical composition of the site this resulting in species-specific sites [35,36]. In the laboratory experiments conducted using different concentration of NPK fertilizers in cups no clear trend in the preference of Cx quinquefasciatus gravid females nor An. coluzzii females to specific concentration of the fertilizer was recorded. The results could derive from the limited number of replicates conducted. The presence of organic matters or microbial organisms in water collections was reported to release chemical cues that could be captured by gravid females to locate suitable oviposition sites [39,40]. During oviposition, females were reported to look for suitable breeding places by analyzing specific cues called semio-chemicals signals such as volatile substances released from the breeding site and which are driving potential olfactory cues mediating oviposition [39,40]. Sites full of organic matters were reported to register high oviposition rate and high egg hatch and to provide enough food for mosquito larvae [41,42]. The fact that mosquito oviposition behavior was not affected by olfactic cues could derive from the low concentration of the NPK used or the fact that the compound were freshly prepared and the absence of bacteria in the milieu to break down organic particles. Yet, sites full of organic matters while attracting females for oviposition could be subject to intense interspecific competitions which could affect the fitness and the development of species.
Fitness studies indicated that the larval development time for Cx quinquefasciatus was shorter in trail containing high NPK concentrations. Similar observations were recorded for Culex pipiens pipiens exposed to organics fertilizers (urea) [43,44]. An. coluzzii larvae on the other side were found to display similar larval development time when reared in trail with different NPK concentrations. The following suggest increase tolerance of An. coluzzii population to organic pollutants and is in line with studies conducted in the city of Yaoundé suggesting high tolerance of this mosquito to organic pollutants [45]. Yet further studies are still needed to assess how this adaptation capacity is affecting mosquito fitness.
The present study provided additional information on the bionomic of An. coluzzii and Cx quinquefasciatus populations. These information could be key for improving control strategies against these vector species in Cameroon.
Acknowledgements
This work received financial support from Wellcome Trust senior Fellowship in Public Health and Tropical Medicine (202687/Z/16/Z) to CAN. The funding body did not had any role in the design, collection of data, analysis and interpretation of data and in writing of the manuscript. The authors thank Djamouko-Djonkam landre for his technical assistance.
Author contribution:
Djiappi-Tchamen B: Data Curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – Original Draft Preparation; Nchoutpouen E: Data Curation, Investigation, Writing – Review & Editing; Talipouo A: Writing – Review & Editing; NKahe D: Investigation, Writing – Review & Editing; Roland B: Investigation, Writing – Review & Editing; Formal Analysis, Software; Kopya E: Investigation, Methodology; Awono-Ambene P: Writing – Review & Editing; Tchuinkam T: Administration, Writing – Review & Editing; Antonio-Nkondjio C: Conceptualization, Funding Acquisition, Project Administration, Supervision, Validation, Visualization, Writing – Original Draft Preparation, Writing – Review & Editing
Competing interests:
No competing interests were disclosed
References
- Rueda LM. Global diversity of mosquitoes (Insecta: Diptera: Culicidae) in freshwater. InFreshwater Animal Diversity Assessment (2007): pp. 477-487. Springer, Dordrecht.
- Snr S, Norma-Rashid Y, Sofian-Azirun M. Mosquitoes larval breeding habitat in urban and suburban areas, Peninsular Malaysia. World Acad Sci Eng Technol 58 (2011): 569-573.
- Antonio-Nkondjio C, Ndo C, Njiokou F, et al. Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasites & Vectors 12 (2019): 501.
- Fontenille D, Wanji S, Djouaka R, et al. Anopheles hancocki, vector secondaire du paludisme au Cameroun. Bulletin de Liaison et de Documentation-OCEAC 33 (2000): 23-26.
- Tonye SG, Kouambeng C, Wounang R, et al. Challenges of DHS and MIS to capture the entire pattern of malaria parasite risk and intervention effects in countries with different ecological zones: the case of Cameroon. Malaria Journal 17 (2018): 156.
- Bartholomay LC, Waterhouse RM, Mayhew GF, et al. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science 330 (2010): 88-90.
- Samy AM, Elaagip AH, Kenawy MA, et al. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PloS One 11 (2016): e0163863.
- Nchoutpouen E, Talipouo A, Djiappi-Tchamen B, et al. Culex species diversity, susceptibility to insecticides and role as potential vector of lymphatic filariasis in the city of Yaoundé, Cameroon. PLoS Neglected Tropical Diseases 133 (2019): e0007229.
- Al Ashry HA, Kenawy MA, Shobrak M. Ecological aspects of the Bancroftian filariasis vectors, Culex pipiens and Cx. quinquefasciatus (Diptera: Culicidae) in Hail. Saudi Arabia. Int J Mosq Res 5 (2018): 25-32.
- Schmid S, Dinkel A, Mackenstedt U, et al. Avian malaria on Madagascar: bird hosts and putative vector mosquitoes of different Plasmodium lineages. Parasites & Vectors 10 (2017): 1-7.
- Richards SL, Mores CN, Lord CC, et al. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector-Borne and Zoonotic Diseases 7 (2007): 629-636.
- Nana-Djeunga HC, Tchatchueng-Mbougua JB, Bopda J, et al. Mapping of Bancroftian filariasis in Cameroon: prospects for elimination. PLoS Negl Trop Dis 9 (2015): e0004001.
- Ngadvou D, Younoussa L, Yonki B, et al. Diversity and spatiotemporal distribution of mosquito species in Ngaoundere, Cameroon 7 (2020): 48–54.
- Brottes H, Rickenbach A, Bres P, Salaun J-J and Ferrara L P. Les arbovirus au Cameroun. Bulletin, Organisation Mondiale de la Santé 35 (1966): 811-825.
- Salaün JJ, Rickenbach A, Brès P, et al. Les arbovirus isolés à partir de moustiques au Cameroun. Bulletin of the World Health Organization 41 (1969): 233.
- Antonio-Nkondjio C, Sonhafouo-Chiana N, Ngadjeu CS, et al. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasites & Vectors 10 (2017): 472.
- WHO | World Malaria Report 2015 [Internet]. [cited 2020 Sep 4]. Available from: https://www.who.int/malaria/publications/world-malaria-report-2015/report/en/
- Bamou R, Mbakop LR, Kopya E, et al. Changes in malaria vector bionomics and transmission patterns in the equatorial forest region of Cameroon between 2000 and 2017. Parasites & Vectors 11 (2018): 464.
- Noreen I, Khan IA, Khater E, et al. Field evaluation of lethal ovitraps for the control of Dengue vectors in Islamabad, Pakistan. International Journal of Ecotoxicology and Ecobiology 2 (2017): 16-25.
- Antonio-Nkondjio C, Fossog BT, Ndo C, et al. Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaoundé (Cameroon): influence of urban agriculture and pollution. Malaria Journal 10 (2011): 154.
- Antonio-Nkondjio C, Youmsi-Goupeyou M, Kopya E, et al. Exposure to disinfectants (soap or hydrogen peroxide) increases tolerance to permethrin in Anopheles gambiae populations from the city of Yaoundé, Cameroon. Malaria Journal 13 (2014): 296.
- Nwane P, Etang J, Chouaibou M, et al. Trends in DDT and pyrethroid resistance in Anopheles gambiaes. s. populations from urban and agro-industrial settings in southern Cameroon. BMC Infectious Diseases 9 (2009): 163.
- Mazigo HD, Mboera LE, Rumisha SF, et al. Malaria mosquito control in rice paddy farms using biolarvicide mixed with fertilizer in Tanzania: semi-field experiments. Malaria Journal 18 (2019): 226.
- Van Den Berg H, Von Hildebrand A, Ragunathan V, et al. Reducing vector-borne disease by empowering farmers in integrated vector management. Bulletin of the World Health Organization 85 (2007): 561-566.
- Coffinet T, Rogier C. Evaluation of the anopheline mosquito aggressivity and of malaria transmission risk: methods used in French Army. Medecine Tropicale: Revue du Corps de Sante Colonial 69 (2009): 109-122.
- Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malaria Journal 19 (2020): 1-20.
- Santolamazza F, Mancini E, Simard F, et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malaria Journal 7 (2008): 163.
- Millar JG, Chaney JD, Mulla MS. Identification of oviposition attractants for Culex quinquefasciatus from fermented Bermuda grass infusions. Journal of the American Mosquito Control Association 8 (1992): 11-17.
- Darriet F, Corbel V. Influence des engrais de type NPK sur l’oviposition d’Aedes aegypti. Parasite 15 (2008): 89-92.
- De Mendiburu F. Agricolae: statistical procedures for agricultural research. R Package Version 1 (2014).
- Pinheiro J, Bates D, DebRoy S, et al. R Core Team. 2018. nlme: linear and nonlinear mixed effects models. R package version 3.1-137. R Found Stat Comput Retrieved from https://CRAN R-project org/package= nlme (2018).
- Huang J, Walker ED, Otienoburu PE, et al. Laboratory tests of oviposition by the African malaria mosquito, Anopheles gambiae, on dark soil as influenced by presence or absence of vegetation. Malaria Journal 5 (2006): 1-5.
- Huang J, Walker ED, Vulule J, et al. The influence of darkness and visual contrast on oviposition by Anopheles gambiae in moist and dry substrates. Physiological Entomology 32 (2007): 34-40.
- Gouagna LC, Robert V. Les anophèles perçoivent-ils les couleurs?. Bulletin de Liaison et de Documentation-OCEAC 26 (1993): 73-74.
- Day JF. Mosquito oviposition behavior and vector control. Insects 7 (2016): 65.
- Lounibos LP. Mosquito breeding and opposition stimulant in fruit husks. Ecological Entomology 3 (1978): 299-304.
- Ponnusamy L, Xu N, Nojima S, et al. Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proceedings of the National Academy of Sciences 105 (2008): 9262-9267.
- Ponnusamy L, Wesson DM, Arellano C, et al. Species composition of bacterial communities influences attraction of mosquitoes to experimental plant infusions. Microbial Ecology 59 (2010): 158-173.
- Craig TP, Itami JK, Price PW. A strong relationship between oviposition preference and larval performance in a shoot-galling sawfly. Ecology 70 (1989): 1691-1699.
- Himeidan YE, Temu EA, El Rayah EA, et al. Chemical cues for malaria vectors oviposition site selection: challenges and opportunities. Journal of Insects 2013 (2013).
- Isoe J, Millar JG, Beehler JW. Bioassays for Culex (Diptera: Culicidae) mosquito oviposition attractants and stimulants. Journal of Medical Entomology 32 (1995): 475-483.
- Souza RS, Virginio F, Riback TI, et al. Microorganism-based larval diets affect mosquito development, size and nutritional reserves in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Frontiers in Physiology 10 (2019): 152.
- Darriet F, Zumbo B, Corbel V, et al. Influence of plant matter and NPK fertilizer on the biology of Aedes aegypti (Diptera: Culicidae). Parasite (Paris, France) 17 (2010): 149-154.
- Olayemi IK, Maduegbuna EN, Ukubuiwe AC, et al. Laboratory studies on developmental responses of the filarial vector mosquito, Culex pipiens pipiens (Diptera: Culicidae), to urea fertilizer. Journal of Medical Sciences 12 (2012): 175.
- Tene BF, Poupardin R, Costantini C, et al. Resistance to DDT in an urban setting: common mechanisms implicated in both M and S forms of Anopheles gambiae in the city of Yaoundé Cameroon. PloS One 8 (2013): e61408.
