Ovarian Growth Factor Protein Expression in Pediatric Patients before and after Cytotoxic Therapy
Article Information
Roni Prag Rosenberg1?, Maayan Maor1?#, Benjamin Fisch1,2, Irit Ben-Aharon3,4, Galia Oron1, Shifra Ash5, Eli Anuka6, Isaac Yaniv5, Enrique Freud7, Miriam Ben Arush8, Fatme Mhameed1, Avi Ben-Haroush1, Yoel Shufaro1,2, Ronit Abir1,2*
1IVF and Infertility Unit, Beilinson Women Hospital, Rabin Medical Center, Petach Tikvah, 4941492 Israel, and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
2Felsenstein Medical Research Center, Beilinson Hospital, Petach Tikvah, 4841492, and Sackler Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel
3Institute of Oncology, Davidoff Cancer Center, Beilinson Rabin Medical Center, Petach Tikvah, Israel and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
4Division of Oncology, Rambam Health Care Campus, Haifa, Israel
5Department of Pediatric Hematology Oncology, Schneider Children’s Medical Center of Israel, Petach Tikvah, 4920235, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
6Department of Biological Chemistry, The Alexander Silberman Institute of Life Sciences, The Hebrew University of Jerusalem, Jerusalem, Israel
7Division of Pediatric Hematology Oncology, Ruth Rappaport Children's Hospital, Rambam Health Care Campus, Haifa, Israel
8Department of Pediatric Surgery, Schneider Children’s Medical Center of Israel, Petach Tikvah, 4920235, Israel, and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
?The authors consider that the first two authors should be regarded as joint
#M. Maor conducted the immunohistochemical studies as partial requirements for her MD degree at Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
*Corresponding Author: Prof. Ronit Abir, IVF and infertility Unit, Beilinson Women Hospital, Rabin Medical Center, Petach Tikvah, 4920235, Israel
Received: 01 May 2020; Accepted: 19 May 2020; Published: 01 July 2020
Citation: Roni Prag Rosenberg, Maayan Maor, Benjamin Fisch, Irit Ben-Aharon, Galia Oron, Shifra Ash, Eli Anuka, Isaac Yaniv, Enrique Freud, Miriam Ben Arush, Fatme Mhameed, Avi Ben-Haroush, Yoel Shufaro, Ronit Abir. Ovarian Growth Factor Protein Expression in Pediatric Patients before and after Cytotoxic Therapy. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 154-183.
View / Download Pdf Share at FacebookAbstract
The improved survival of young cancer patients results often in follicle destruction. The infertility level varies by age, with alkylating agents considered the most gonadotoxic. One option for fertility preservation is ovarian cryostorage, often conducted post-chemotherapy. Large number of follicles remain in ovaries of pediatric patients post-chemotherapy. Anti-Mullerian hormone (AMH), growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) are ovarian markers. Their ovarian expression in post-chemotherapy girls might provide an indication of follicle normality, and if it is worthwhile to cryopreserve such tissue. AMH, GDF9 and BMP15 protein expression in human ovarian samples from 49 girls pre-and-post-chemotherapy was evaluated by regular immunohistochemistry (IHC) and immunofluorescence (IF) for confocal laser microscopy. Protein expression for the three growth factors in follicles of pediatric patients pre-and-post-chemotherapy (including alkylating agent exposure) was identified by both methods from primordial stages onwards in oocytes and granulosa cells, and in irregularly shaped follicles. In the pre-chemotherapy group, IHC AMH staining seemed strongest at age ≤ 8 years, and was more abundant in pubertal-than-pre-pubertal patients by IF. Post-chemotherapy patients had significantly more immunostaining for AMH and BMP15 in atretic follicles after exposure to alkylating agents. IHC immunostaining for all three proteins seemed fuller before than after chemotherapy; there were more follicles containing growth factor-expressing granulosa cells post-chemotherapy; BMP15 staining seemed stronger pre-than-post-chemotherapy. The growth factor distribution post-chemotherapy as well as the high numbers of remaining follicles, suggests that it is worthwhile to cryostore ovarian tissue from pediatric patients even after chemotherapy initiation, including alkylating agent
Keywords
Fertility preservation; Anti-Mullerian hormone (AMH); Growth differentiation factor 9 (GDF9); Bone morphogenetic protein 15 (BMP15); Immunohistochemistry; Immunofluorescence for confocal laser microscopy
Fertility preservation articles, Anti-Mullerian hormone (AMH) articles, Growth differentiation factor 9 (GDF9) articles, Bone morphogenetic protein 15 (BMP15) articles, Immunohistochemistry articles, Immunofluorescence for confocal laser microscopy articles
Fertility preservation articles Fertility preservation Research articles Fertility preservation review articles Fertility preservation PubMed articles Fertility preservation PubMed Central articles Fertility preservation 2023 articles Fertility preservation 2024 articles Fertility preservation Scopus articles Fertility preservation impact factor journals Fertility preservation Scopus journals Fertility preservation PubMed journals Fertility preservation medical journals Fertility preservation free journals Fertility preservation best journals Fertility preservation top journals Fertility preservation free medical journals Fertility preservation famous journals Fertility preservation Google Scholar indexed journals Anti-Mullerian hormone (AMH) articles Anti-Mullerian hormone (AMH) Research articles Anti-Mullerian hormone (AMH) review articles Anti-Mullerian hormone (AMH) PubMed articles Anti-Mullerian hormone (AMH) PubMed Central articles Anti-Mullerian hormone (AMH) 2023 articles Anti-Mullerian hormone (AMH) 2024 articles Anti-Mullerian hormone (AMH) Scopus articles Anti-Mullerian hormone (AMH) impact factor journals Anti-Mullerian hormone (AMH) Scopus journals Anti-Mullerian hormone (AMH) PubMed journals Anti-Mullerian hormone (AMH) medical journals Anti-Mullerian hormone (AMH) free journals Anti-Mullerian hormone (AMH) best journals Anti-Mullerian hormone (AMH) top journals Anti-Mullerian hormone (AMH) free medical journals Anti-Mullerian hormone (AMH) famous journals Anti-Mullerian hormone (AMH) Google Scholar indexed journals Growth differentiation factor 9 (GDF9) articles Growth differentiation factor 9 (GDF9) Research articles Growth differentiation factor 9 (GDF9) review articles Growth differentiation factor 9 (GDF9) PubMed articles Growth differentiation factor 9 (GDF9) PubMed Central articles Growth differentiation factor 9 (GDF9) 2023 articles Growth differentiation factor 9 (GDF9) 2024 articles Growth differentiation factor 9 (GDF9) Scopus articles Growth differentiation factor 9 (GDF9) impact factor journals Growth differentiation factor 9 (GDF9) Scopus journals Growth differentiation factor 9 (GDF9) PubMed journals Growth differentiation factor 9 (GDF9) medical journals Growth differentiation factor 9 (GDF9) free journals Growth differentiation factor 9 (GDF9) best journals Growth differentiation factor 9 (GDF9) top journals Growth differentiation factor 9 (GDF9) free medical journals Growth differentiation factor 9 (GDF9) famous journals Growth differentiation factor 9 (GDF9) Google Scholar indexed journals Bone morphogenetic protein 15 (BMP15) articles Bone morphogenetic protein 15 (BMP15) Research articles Bone morphogenetic protein 15 (BMP15) review articles Bone morphogenetic protein 15 (BMP15) PubMed articles Bone morphogenetic protein 15 (BMP15) PubMed Central articles Bone morphogenetic protein 15 (BMP15) 2023 articles Bone morphogenetic protein 15 (BMP15) 2024 articles Bone morphogenetic protein 15 (BMP15) Scopus articles Bone morphogenetic protein 15 (BMP15) impact factor journals Bone morphogenetic protein 15 (BMP15) Scopus journals Bone morphogenetic protein 15 (BMP15) PubMed journals Bone morphogenetic protein 15 (BMP15) medical journals Bone morphogenetic protein 15 (BMP15) free journals Bone morphogenetic protein 15 (BMP15) best journals Bone morphogenetic protein 15 (BMP15) top journals Bone morphogenetic protein 15 (BMP15) free medical journals Bone morphogenetic protein 15 (BMP15) famous journals Bone morphogenetic protein 15 (BMP15) Google Scholar indexed journals Immunohistochemistry articles Immunohistochemistry Research articles Immunohistochemistry review articles Immunohistochemistry PubMed articles Immunohistochemistry PubMed Central articles Immunohistochemistry 2023 articles Immunohistochemistry 2024 articles Immunohistochemistry Scopus articles Immunohistochemistry impact factor journals Immunohistochemistry Scopus journals Immunohistochemistry PubMed journals Immunohistochemistry medical journals Immunohistochemistry free journals Immunohistochemistry best journals Immunohistochemistry top journals Immunohistochemistry free medical journals Immunohistochemistry famous journals Immunohistochemistry Google Scholar indexed journals Immunofluorescence for confocal laser microscopy articles Immunofluorescence for confocal laser microscopy Research articles Immunofluorescence for confocal laser microscopy review articles Immunofluorescence for confocal laser microscopy PubMed articles Immunofluorescence for confocal laser microscopy PubMed Central articles Immunofluorescence for confocal laser microscopy 2023 articles Immunofluorescence for confocal laser microscopy 2024 articles Immunofluorescence for confocal laser microscopy Scopus articles Immunofluorescence for confocal laser microscopy impact factor journals Immunofluorescence for confocal laser microscopy Scopus journals Immunofluorescence for confocal laser microscopy PubMed journals Immunofluorescence for confocal laser microscopy medical journals Immunofluorescence for confocal laser microscopy free journals Immunofluorescence for confocal laser microscopy best journals Immunofluorescence for confocal laser microscopy top journals Immunofluorescence for confocal laser microscopy free medical journals Immunofluorescence for confocal laser microscopy famous journals Immunofluorescence for confocal laser microscopy Google Scholar indexed journals gonadotoxic articles gonadotoxic Research articles gonadotoxic review articles gonadotoxic PubMed articles gonadotoxic PubMed Central articles gonadotoxic 2023 articles gonadotoxic 2024 articles gonadotoxic Scopus articles gonadotoxic impact factor journals gonadotoxic Scopus journals gonadotoxic PubMed journals gonadotoxic medical journals gonadotoxic free journals gonadotoxic best journals gonadotoxic top journals gonadotoxic free medical journals gonadotoxic famous journals gonadotoxic Google Scholar indexed journals post-chemotherapy articles post-chemotherapy Research articles post-chemotherapy review articles post-chemotherapy PubMed articles post-chemotherapy PubMed Central articles post-chemotherapy 2023 articles post-chemotherapy 2024 articles post-chemotherapy Scopus articles post-chemotherapy impact factor journals post-chemotherapy Scopus journals post-chemotherapy PubMed journals post-chemotherapy medical journals post-chemotherapy free journals post-chemotherapy best journals post-chemotherapy top journals post-chemotherapy free medical journals post-chemotherapy famous journals post-chemotherapy Google Scholar indexed journals ovarian cryostorage articles ovarian cryostorage Research articles ovarian cryostorage review articles ovarian cryostorage PubMed articles ovarian cryostorage PubMed Central articles ovarian cryostorage 2023 articles ovarian cryostorage 2024 articles ovarian cryostorage Scopus articles ovarian cryostorage impact factor journals ovarian cryostorage Scopus journals ovarian cryostorage PubMed journals ovarian cryostorage medical journals ovarian cryostorage free journals ovarian cryostorage best journals ovarian cryostorage top journals ovarian cryostorage free medical journals ovarian cryostorage famous journals ovarian cryostorage Google Scholar indexed journals ovarian tissue articles ovarian tissue Research articles ovarian tissue review articles ovarian tissue PubMed articles ovarian tissue PubMed Central articles ovarian tissue 2023 articles ovarian tissue 2024 articles ovarian tissue Scopus articles ovarian tissue impact factor journals ovarian tissue Scopus journals ovarian tissue PubMed journals ovarian tissue medical journals ovarian tissue free journals ovarian tissue best journals ovarian tissue top journals ovarian tissue free medical journals ovarian tissue famous journals ovarian tissue Google Scholar indexed journals
Article Details
Abbreviations:
AMH- Anti-Mullerian hormone; GDF9- Growth differentiation factor 9; BMP15- Bone morphogenetic protein 15; IHC- Immunohistochemistry; IF- Immunofluorescence; GC- Granulosa cell
1. Introduction
The improved survival of patients with childhood malignancies [1, 2] has led clinicians to address the long-term adverse effect of cytotoxic agents on ovarian reserve [2-4]. The level of infertility varies in chemotherapy-exposed patients by age, with ovarian failure being less severe in younger patients and treatment duration, dose and type, with alkylating agents considered the most gonadotoxic [2, 3, 5]. One option for fertility preservation in cancer patients is cryopreservation of ovarian tissue; a procedure that cannot be always conducted before anti-cancer therapy. So far, over 130 live-births have been reported after transplantation of frozen-thawed ovarian tissue to cancer survivors [4, 6], including two frozen at childhood [7, 8]. Although large number of follicles remain in ovaries of pediatric follicles even after chemotherapy [3, 5], transmission electron microscopy studies demonstrated post-chemotherapy intracellular changes in both adults and children, including an abnormally thick basal lamina surrounding the follicles, oocyte vacuolization and reduction in normal granulosa cell (GC) nuclei [3, 9]. Anti-Mullerian hormone (AMH) [10], growth differentiation factor 9 (GDF9) [11] and bone morphogenetic protein 15 (BMP15) [12], all members of the transforming growth factor beta (TGFβ) superfamily of follicles play an important role in various stages of folliculogenesis [13-22]. AMH, also termed Mullerian-inhibiting substance (MIS) is a dimeric glycoprotein [10], produced by GC [23] and expressed from primary follicular stages onwards [10] AMH levels serve as markers of ovarian follicular reserve, also after chemotherapy [5, 24]. It has been shown to inhibit primordial follicle recruitment in mice [25], but its role in primates, including humans, is probably more complex [26-28]. AMH protein expression was identified in human ovaries from fetuses, from girls before puberty to various pubertal stages [29], and from regularly cycling women [23]. The highest AMH levels were present in GCs of secondary and antral follicles.
GDF9 and BMP15 are closely related structurally to each other [12, 16, 17]. Both proteins have six cysteine residues, and not seven as in other TGFβ family members [21], and both are expressed and produced in oocytes [11, 21, 30]. GDF9 protein expression was identified in the cytoplasm and nucleus of oocytes and in GCs of girls and the women [11], and the GDF9 protein as well as mRNA transcripts were demonstrated in ovaries from women [11, 31, 32]. GDF9 protein expression in both oocytes and GCs increases gradually with follicular development, reaching a peak in antral follicles [32]. BMP15, also called GDF9B, is encoded by an X-linked gene [12, 17, 20]. It is a paracrine signaling molecule involved in oocyte-and-follicular development [12, 21]. In a study that included ovaries from girls and women, BMP15 protein expression was identified already from the primordial follicle stage in oocytes, GCs and stroma cells of girls and women, and the mRNA transcripts were detected in all ovarian samples from girls [12]. It is unknown if AMH, GDF9, and BMP15 are expressed in follicles of Pediatric patients after anti-cancer therapy.
The Balbiani body is a cytoplasmic structure present in young immature oocytes also from humans [33] made up of a transient collection of mitochondria, Golgi complexes, endoplasmic reticulum, and electron-dense granulofibrillar material. It assembles in an asymmetrical fashion adjacent to the nucleus of immature oocytes. The composition of the Balbiani body appears to be dynamic, and its regulation and function remain largely obscure [33, 34]. One more recent study suggested that the Balbiani body might be involved in selection/elimination of dysfunctional mitochondria from female germline cells [35]. It is unknown if AMH, GDF9, and BMP15 are expressed in the region of the Balbiani body. As AMH, GDF9 and BMP15 are known markers of normal ovaries and ovarian reserve [13, 22], the aim of this study was to examine and compare between the protein expression of these three growth factors in follicles of young girls before and after administration of cytotoxic therapy; to compare their expression after different anti-cancer treatments (alkylating vs. non-alkylating agents); patient age and pubertal status. The expression of AMH, GDF9 and BMP15 in ovaries of young girls after chemotherapy might provide an additional indication of the remainder of normal follicles, and if it is worthwhile to cryopreserve such tissue [3, 5].
2. Materials and Methods
2.1 Ovarian material
The Ethics Committee of Rabin Medical Center approved the study protocol (RMC-10-100), and informed consent was obtained from the parents of the minors. The cohort included 49 girls who had undergone laparoscopy for cryopreservation of ovarian tissue (Tables IA and IB). Patients were either before chemotherapy (chemotherapy naïve, pre-chemotherapy group, Table IA) or after chemotherapy (post-chemotherapy group, Table IB). There was no overlap of patients between the groups.
|
Patients no. |
Age |
Disease |
Normal follicles |
IHC staining |
IF staining |
|
1 |
2 |
AML |
+ |
+ |
+ |
|
2 |
3 |
Medulloblastoma |
+ |
+ |
|
|
3 |
3 |
Brain tumor |
+ |
+ |
+ |
|
4 |
5 |
Rhabdomyosarcoma |
+ |
+ |
|
|
5 |
5 |
ALL |
+ |
+ |
|
|
6 |
5 |
Myelodysplastic syndrome |
+ |
||
|
7 |
7 |
Beta Thalassemia |
+ |
+ |
+ |
|
8 |
8 |
Rhabdomyosarcoma |
+ |
+ |
|
|
9 |
8 |
Abdominal Mesothelioma |
+ |
+ |
|
|
10 |
9 |
Medulloblastoma |
+ |
+ |
+ |
|
11 |
10 |
Ewing's sarcoma |
+ |
+ |
|
|
12 |
11 |
Ewing's sarcoma |
+ |
+ |
|
|
13 |
12 |
Brain tumor |
+ |
+ |
|
|
14 |
12 |
Medulloblastoma |
+ + + |
+ |
|
|
15 |
12 |
Primitive Neuroectodermal Tumour |
+ |
+ |
|
|
16 |
13 |
Ewing's sarcoma |
+ |
+ |
|
|
17 |
13 |
Osteosarcoma |
+ |
+ |
+ |
|
18 |
13 |
Osteosarcoma |
+ |
+ |
|
|
19 |
13 |
HL |
+ |
+ |
+ |
|
20 |
14 |
Ewing's sarcoma |
+ |
+ |
|
|
21 |
15 |
Nasopharyngeal Sarcoma |
+ |
+ |
|
|
22 |
16 |
HL |
+ |
+ |
|
|
23 |
16 |
HL |
+ |
+ |
|
|
24 |
16 |
Osteosarcoma |
+ |
+ |
|
|
25 |
16 |
ALL |
+ |
+ |
|
|
26 |
16 |
Ewing's sarcoma |
+ |
+ |
+ |
|
27 |
17 |
HL |
+ |
+ |
|
|
28 |
17 |
HL |
+ |
+ |
+ |
|
29 |
17 |
HL |
+ |
+ |
+ |
|
30 |
17 |
Vaginal Ewing sarcoma |
+ |
+ |
|
|
31 |
18 |
Lupus Nephritis |
+ |
+ |
|
|
31 Patients* |
11.5±5** |
*Total patient number; **Average-SD in years; Note: IHC= Immunohistochemistry; IF=Immunofluorescence;
AML= Acute myeloid leukemia; ALL=Acute lymphoblastic leukemia; HL=Hodgkin's lymphoma
Table 1A: Details of the chemotherapy naïve patients and type of staining.
|
Patients no. |
Age |
Disease |
Prior treatment |
Normal follicles |
IHC |
IF |
|
32 |
2 |
Neuroblastoma |
Alkylating agents |
+ |
+ |
|
|
33 |
3 |
ALL |
Alkylating agents |
+ |
+ |
+ |
|
34 |
4 |
Wilms tumor |
Alkylating agents |
+ |
+ |
|
|
35 |
5 |
optic nerve tumor |
Carboplatin, Vinblastine, Vincristine, Bevacizumab |
+ |
+ |
|
|
+ |
||||||
|
36 |
5 |
AML |
Alkylating agents |
+ |
+ |
|
|
37 |
8 |
HL |
ABVD +chest irradiation |
+ |
+ |
|
|
38 |
9 |
Brain glioma |
Bevacizumab |
+ |
+ |
|
|
39 |
11 |
ALL |
Alkylating agents |
+ |
+ |
|
|
40 |
11 |
Eye sarcoma |
Alkylating agents |
+ |
+ |
|
|
41 |
13 |
Germ cell tumor |
BEP |
+ |
+ |
+ |
|
42 |
14 |
AML |
Doxo+VP16+cytarabine |
+ |
+ |
|
|
43 |
15 |
ALL |
Alkylating agents |
+ |
+ |
|
|
44 |
16 |
AML |
Alkylating agents |
+ |
+ |
+ |
|
45 |
16 |
AML |
Alkylating agents |
+ |
+ |
|
|
46 |
16 |
NHL |
ABVD |
+ |
+ |
|
|
47 |
17 |
HL |
Alkylating agents |
+ |
+ |
|
|
48 |
18 |
HL |
ABVD |
+ |
+ |
|
|
49 |
18 |
HL |
ABVD |
+ |
+ |
|
|
18 Patients* |
11±5.5** |
Note: *total patient number, **Average-standard deviation (SD), AML= Acute myeloid leukemia; ALL=Acute lymphoblastic leukemia HL=Hodgkin's lymphoma; NHL= Non-Hodgkin lymphoma; BEP=bleomycin, etopside, cisplatin; oxo=doxorubicin; VP16= etopside; ABVD= doxorubicin, bleomycin, vinblastin, decarbazine
Table 1B: Details of the post-chemotherapy patients and type of staining.
2.2 Histological preparation for light microscopy
Uniform sized ovarian tissue specimens (~5mmX5mm) were fixed and prepared for paraffin embedding and sectioning [11,12]. The number of follicles was counted throughout the field (magnification X100) in two levels per specimen (with at least 50 µm between levels to avoid counting the same follicle twice), this method was used by us previously and is reliable for evaluation of follicle number distribution. Unstained sections were placed on OptiPlus positive-charged microscope slides for IHC and IF studies.
2.3 IHC for AMH, GDF9 and BMP15
The sections were dehydrated, microwaved with citrate buffer at pH 6 (CheMate buffer, DAKOCytomation, Glostrup, Denmark) for antigen retrieval and quenched with hydrogen peroxide (Gadot, Binyamina, Israel) [11, 22]. They were then incubated overnight with goat polyclonal antibodies against AMH (MIS, Santa Cruz Biotechnology, Dallas, Texas, USA, sc-6886, diluted 1/80 and 1/100), GDF9 (Santa Cruz Biotechnology, sc-12244, diluted 1/60 and 1/100), and BMP15 (GDF9B, Santa Cruz Biotechnology, sc-18337, diluted 1/60 and 1/100) or with a negative control solution of combinations of the relevant primary antibodies absorbed with their respective blocking peptides (sc-6886P, sc-12244P, sc-18337P, respectively). The following morning, the samples were incubated with horseradish peroxidase polymer conjugate against goat antibodies (Zymed Laboratories Inc., San Francisco, CA, USA) and stained with 3-amino-9-ethylcarbazole (Zymed Laboratories Inc.) resulting in red-brown staining AMH, GDF9 and BMP15 expression with blue Mayer’s hematoxylin (Pioneer Research Chemicals Ltd., Colchester Essex, UK) counterstaining. The slides were mounted with a water-based mounting medium (Fluoromount, Diagnostic BioSystems, Pleasanton, CA, USA). The follicles were counted using a computerized image analyser (analySIS, Soft Imaging System, Digital Solutions for Imaging and Microscopy, System GmbH, Munster, Germany), and classified according to Gougeon [41] primordial follicles (30-50 mm in diameter) oocytes are surrounded by a single layer of flattened somatic GC; primary follicles (50-80 mm in diameter) after primordial follicle activation to cuboidal GCs; secondary follicles (80 mm-0.2 mm in diameter) with increased GC proliferation rate with consequent multilaminar layer formation and a theca layer surrounding the GCs, antral follicles (early antral follicle: 0.2-0.4 mm in diameter), final follicular stage-follicle: follicle contains a fluid-filled cavity within several cuboidal GC layers. Atretic follicles were characterized by pyknotic cells, eosinophilia of the ooplasm, and clumping of the chromatin material.
The strength of the immunostaining was indicated as weak, medium or, strong [11, 12]. Staining in the oocyte cytoplasm was defined as full (in the whole cytoplasm) or partial (in a part of the cytoplasm).
2.4 IF for confocal laser microscopy
The sections were dehydrated [36], and placed in a microwave with tris ethylenediaminetetraacetic acid (Tris-EDTA) buffer at pH 9.0 (diluted from a X10 concentrated Tris-EDTA buffer at pH 9.0, Novus Biologicals, Littleton, CO, USA) to enhance antigen retrieval. Thereafter, the slides were incubated with the blocking buffer consisting of phosphate buffer saline (PBS, Biological Industries, Beit Ha'emek, Israel) combined with 4% serum substitute supplement (Irvine Scientific, Santa Ana, CA, USA), 0.05% Tween 20 (Sigma, St Louis, MO, USA), and 0.3% triton (Sigma), and incubated overnight with a solution of the diluted primary antibodies. We initially used a four-color IF staining system (with three primary antibodies) that included incubation with the three diluted primary antibodies (all at a concentration of 1/200) in equal volumes: mouse monoclonal antibodies against MIS (Santa Cruz Biotechnology, sc-377140), rabbit polyclonal antibodies against GDF9 (Santa Cruz Biotechnology, sc-366853) and goat polyclonal antibodies against GDF9B (Santa Cruz Biotechnology, sc-18337) diluted with the blocking buffer. The negative controls included a combination of ChromoPure goat IgG, whole molecule (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), normal mouse IgG (Santa Cruz Biotechnology, sc-2025) and normal rabbit IgG (Santa Cruz Biotechnology, sc-2027) (all at a concentration of 1/200) in equal volume [37].
However, this combination of four-color IF staining system (with three primary antibodies) resulted in most cases in low color intensity, so we changed the primary antibodies and used mostly a three-color system (with two primary antibodies, also at a concentration of 1/200) in equal volumes as follows: (I) GDF9 and AMH - goat polyclonal antibodies against GDF9 (Santa Cruz Biotechnology, sc-12244) and rabbit polyclonal antibodies against MIS (Santa Cruz Biotechnology, sc-28912). The negative controls included a combination of ChromoPure goat IgG, whole molecule (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and normal rabbit IgG (Santa Cruz Biotechnology, sc-2027) (all at a concentration of 1/200) in equal volume [37]. (II) BMP15 and AMH - goat polyclonal antibodies against GDF9B (Santa Cruz Biotechnology, sc-18337) and rabbit polyclonal antibodies against MIS (Santa Cruz Biotechnology, sc-28912). The negative controls included the same goat and rabbit antibody combinations as in (I). (III) BMP15 and AMH - mouse monoclonal antibodies against GDF9B (Santa Cruz Biotechnology, sc-271824) and rabbit polyclonal antibodies against MIS (Santa Cruz Biotechnology, sc-28912). The negative controls included a combination of normal mouse IgG (Santa Cruz Biotechnology, sc-2025) and normal rabbit IgG (Santa Cruz Biotechnology, sc-2027) (all at a concentration of 1/200) in equal volume [37].
The following morning the sections were further incubated with a solution of the secondary antibodies suitable for the relevant primary antibodies diluted with the blocking buffer in equal volumes (all at a concentration of 1/200): Cy 5-conjugated donkey anti-mouse antibodies (AffiniPure, Jackson Laboratories, West Grove, PA, USA, 715-175-151), biotin-SP conjugated donkey anti rabbit antibodies (AffiniPure, Jackson Laboratories, 711-065-152) and Alexa Fluor 488-conjugated donkey anti goat antibodies (AffiniPure, Jackson Laboratories, 705-545-147), respectively. Initially all the secondary antibodies were mixed together for the four-color immunofluorescent staining system but, as above, further studies included combinations of two antibodies each time, as follows:
(I) Alexa Fluor 488-conjugated donkey anti goat antibodies (AffiniPure, Jackson Laboratories, 705-545-147) and biotin-SP conjugated donkey anti rabbit antibodies (AffiniPure, Jackson Laboratories, 711-065-152).
(II) Alexa Fluor 488-conjugated donkey anti-goat antibodies (AffiniPure, Jackson Laboratories, 705-545-147) and biotin-SP conjugated donkey anti rabbit antibodies (AffiniPure, Jackson Laboratories, 711-065-152).
(III) Cy 5-conjugated donkey anti-mouse antibodies (AffiniPure, Jackson Laboratories) and biotin-SP conjugated donkey anti rabbit antibodies (AffiniPure, Jackson Laboratories, 711-065-152).
All these secondary antibody combinations were also utilized for the negative controls. To enhance the staining, we further incubated the relevant slides with Cy3-conjugated streptavidin (Jackson Laboratories, 016-160-084) diluted (1/100) with PBS (Biological Industries). A second set of negative controls was prepared for the calibration of the microscope and included sections incubated only with the relevant diluted secondary antibody solutions without primary antibodies. Finally, the samples were incubated with 4',6-diamidino-2-phenylindole (DAPI) background staining (Sigma, D9564) diluted (1/1000) with distilled water (Biological Industries). The slides were mounted with a water-based mounting medium (Fluoromount, Diagnostic BioSystems). The slides were visualized and photographed with a Leica SP8 confocal laser microscope. The non-specific very low fluorescence observed in the negative controls was removed from all the immunofluorescent stained sections. The expression of AMH, GDF9, and BMP15 in the photographs was compared for color distribution and intensity in ovarian cellular sites (oocytes, GCs and stroma cells) from samples before and after chemotherapy, according to chemotherapy protocols, and after exposure to alkylating agents or non-alkylating agents and in follicles from pre-menarchal and post-menarchal girls.
2.5 Statistical analysis
Analysis of variance, chi-square test, and Fisher’s exact test, were used as required. A P value of <0.05 was considered statistically significant. All analyses were performed with StatView 5 (SAS Institute, Cary, North Carolina, USA).
3. Results
In general macrospically the size of the ovaries depended on patient age: the younger the patients were, the smaller their ovaries. Although we did not measure the ovaries, their size seemed more age-dependent than chemotherapy-dependent.
3.1 Ovarian sources for IHC
Ovarian samples retrieved from 40 girls (aged 2-18 years) (Tables IA and IB) were used for IHC staining. Twenty-six girls were chemotherapy naïve (Table IA) and 14 girls had been exposed to various chemotherapy protocols including alkylating agents (Table IB), either recently or following disease recurrence, prior to salvage treatment [5], and we tried to present as much as possible information of their treatment. Two pre-chemotherapy patients had a non-malignant disease before bone marrow transplantation: beta Thalassemia and Lupus Nephritis. There were 17 pre-menarchal girls (Table IA and IB) of whom 11 were chemotherapy-naïve and six after chemotherapy. The remaining 23 girls were post-menarchal of whom 15 were chemotherapy naïve and eight after chemotherapy. There were no significant differences in patient age between the pre-and post-chemotherapy groups.
3.2 IHC results
The immunohistochemical studies identified 1,472 follicles from chemotherapy naïve patients: 910 primordial (62%), 226 primary (15%), 88 secondary (6%), four antral (<1%) and 244 atretic (17%). In addition, 920 follicles from post-chemotherapy patients were examined: 541 primordial (59%), 171 primary (19%), 26 secondary (3%), one antral (<1%), and 181 atretic (20%). Figure 1 shows photographs of the immunohistochemical expression of the various growth factors. It is noteworthy that normal follicles were identified in all sections whether from pre- or post-chemotherapy patients (Table 1A). Table II summarizes the observations of IHC protein expression of AMH, GDF9, and BMP15 in ovarian samples from girls before and after chemotherapy. The expression of all three growth factors was identified from primordial follicles stages onwards in oocytes and GCs. We found no differences in the expression pattern of the growth factors among the classes also among the pre- or post-chemotherapy groups. There was also no difference in stroma cell staining of any of the growth factors before and after chemotherapy.
Compared to the pre-chemotherapy group, the post-chemotherapy group was characterized by significantly more AMH stained atretic follicles (78/349, 22% Vs, 69/462, 15%, P=0.034); significantly more positively AMH-and BMP15 stained irregularly shaped follicles for AMH and BMP15 after exposure to alkylating agents (AMH: 45/143, 31% Vs. 69/462, 15%. BMP15: 37/124, 30% 37/124, 30% Vs. 50/466, 11% P<0.0001 for both), and more BMP15 stained irregularly shaped follicles in the post-chemotherapy group than in the chemotherapy naïve group (close to significant value, P>0.071) (Figures 1A and 1B). Figures 1C and 1D demonstrate follicular IHC staining for AMH. In the pre-chemotherapy group, AMH cytoplasmic staining was the strongest in the eight girls aged <8 years, and then became weaker as patient age increased. Samples from five patients showed only cytoplasmic staining, and three also had GC staining. Figure 1E and 1F demonstrate IHC staining for GDF9, and figures 1G and 1H demonstrate follicular IHC staining for BMP15. In general, BMP15 expression seemed stronger after than before chemotherapy (Table 2). There were no differences in IHC staining pattern of the three growth factors between girls with hematological or solid tumours.
|
Follicular staining+intensity |
AMH |
GDF9 |
BMP15 |
|
Before Therapy |
|||
|
Oocyte |
|||
|
None |
13% |
19% |
|
|
Partial |
47% |
31% |
|
|
Full |
40% |
76% |
50% |
|
Cytoplasm |
|||
|
None |
7% |
24% |
19% |
|
Weak |
40% |
35% |
56% |
|
Weak-medium |
20% |
||
|
Medium |
33% |
35% |
25% |
|
Strong |
6% |
||
|
Nucleus |
|||
|
None |
75% |
41% |
31% |
|
Weak |
6% |
41% |
69% |
|
Medium |
13% |
12% |
|
|
Strong |
6% |
||
|
Granulosa cells |
|||
|
None |
19% |
6% |
13% |
|
Weak |
44% |
29% |
81% |
|
Medium |
31% |
53% |
6% |
|
Strong |
12% |
||
|
Stroma |
Scattered |
Scattered |
Scattered |
|
After Therapy |
|||
|
Oocyte |
|||
|
None |
25% |
29% |
|
|
Partial |
67% |
25% |
14% |
|
Full |
33% |
50% |
57% |
|
Cytoplasm |
|||
|
None |
25% |
14% |
|
|
Weak |
17% |
50% |
14% |
|
Weak-medium |
33% |
33% |
|
|
Medium |
50% |
50% |
71% |
|
Nucleus |
|||
|
None |
67% |
63% |
43% |
|
Weak |
17% |
37% |
29% |
|
Medium |
17% |
29% |
|
|
Granulosa cells |
|||
|
None |
50% |
63% |
29% |
|
Weak |
|||
|
Weak-medium |
50% |
37% |
29% |
|
Medium |
43% |
||
|
Stroma |
Scattered |
Scattered |
Scattered |
Table 2: Immunohistochemical protein expression for AMH, GDF9 and BMP15 in human ovaries before and after chemotherapy.
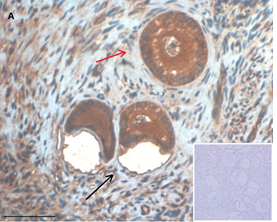
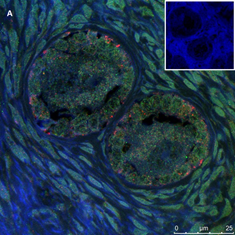
Ovarian section from a 3-year-old girl after therapy (patient no. 33). Note the crescent shaped atretic follicles (black arrow) and the normal primary follicle (red arrow); the red-brown BMP15 staining in the oocyte and GCs. Original magnification X400. Scale bar=30µm at the left lower corner. The primary antibody used was an anti GDF9B antibody (Santa Cruz Biotechnology, sc-18337). The negative control was prepared by absorbing the primary antibody with its blocking peptide (Santa Cruz Biotechnology sc-8337P) and is shown at the bottom right corner. Note the follicles and the exclusive blue hematoxylin staining.
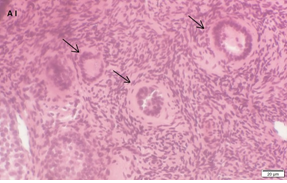
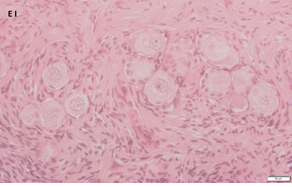
An Hematoxylin and Eosin stained section of the same patient as in 1A. Note the three secondary follicles (arrows).
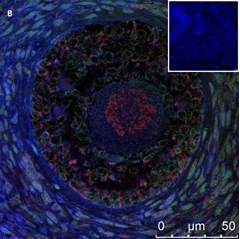
Ovarian section from an 8-year-old girl after therapy (patient no. 37). Note the abnormal follicle without GC; the red-brown BMP15 staining in the oocyte’s cytoplasm and nucleus. Original magnification X400. Scale bar=20µm at the left lower corner. The primary antibody used was an anti GDF9B (Santa Cruz Biotechnology, sc-18337). The negative control was prepared by absorbing the primary antibody with its blocking peptide (Santa Cruz Biotechnology sc-8337P).
An Hematoxylin and Eosin stained section of the same patient as in 1B. Note the primary, possibly atretic follicle (arrow).
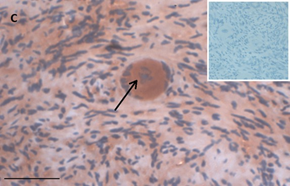
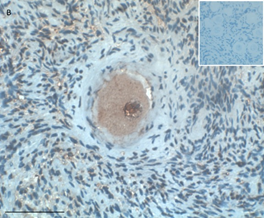
Ovarian section from an 8-year-old girl before therapy (patient no. 9). Note the strong red-brown AMH staining intensity in the oocyte cytoplasm (arrow). Original magnification X400. Scale bar=30µm at the left lower corner. The primary antibody was a goat polyclonal antibody against AMH (MIS, Santa Cruz Biotechnology, sc-6886) The negative control was prepared by absorbing the primary antibody with its blocking peptide (Santa Cruz Biotechnology, sc-6886P). The negative control is in the upper right corner. Note the follicles and the exclusive blue hematoxylin staining.
An Hematoxylin and Eosin stained section of the same patient as in 1C. Note the cluster of primordial and primary follicles.
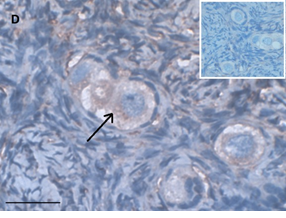
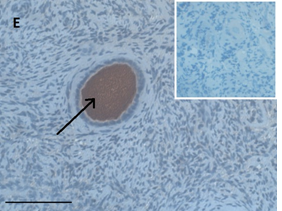
Ovarian section from a 14-years-old girl after therapy (patient no. 42). Note the abnormal follicle with the partial-weak red-brown staining intensity for AMH in the cytoplasm (arrow) and its two oocytes. The primary antibody was a goat polyclonal antibody against AMH (MIS, Santa Cruz Biotechnology, sc-6886), Original magnification X400. Scale bar=30µm on the left lower corner. The negative control is at the upper right corner. The negative control was prepared by absorbing the primary antibody with its blocking peptide (Santa Cruz Biotechnology, sc-6886P). Note the follicles and the exclusive blue hematoxylin staining.
An Hematoxylin and Eosin stained section of the same patient as in 1D. Note the cluster of primordial follicles.
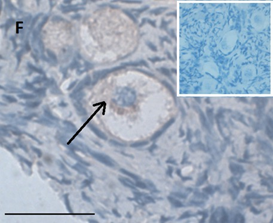
Ovarian section from the same patient as in panel 1C. Note the strong full cytoplasmic staining intensity GDF9 (arrow). The primary antibody was a goat polyclonal antibody against AMH (MIS, Santa Cruz Biotechnology, sc-6886), Scale bar=35µm on the left lower corner. The negative control was prepared by absorbing the primary antibody with its blocking peptide (Santa Cruz Biotechnology, sc-6886P). The negative control is at the upper right corner. Note the follicles and the exclusive blue hematoxylin staining.
An Hematoxylin and Eosin stained section of the same patient as in 1E. Note the Cluster of primordial and primary follicles.
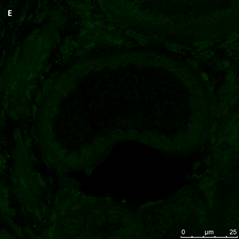
Ovarian section from the same patient as in panel 1C. Note the weak partial cytoplasmic staining intensity for GDF9 (arrow), possibly mostly in the location of the Balbiani body. Original magnification X400. Scale bar=35µm at the left lower corner. The primary antibody used was a goat polyclonal antibody an against GDF9B (Santa Cruz Biotechnology, sc-18337). Scale bar=35µm on the left lower corner. The negative control was prepared by absorbing the primary antibody with its blocking peptide (Santa Cruz Biotechnology sc-8337P). The negative control is in the upper right corner. Note the follicles and the exclusive blue hematoxylin staining.
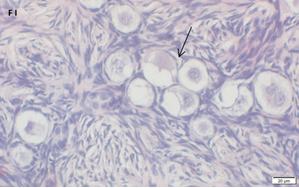
An Hematoxylin and Eosin stained section of the same patient as in 1F. Note the crescent shaped follicles (arrow) and the cluster of primordial and primary follicles.
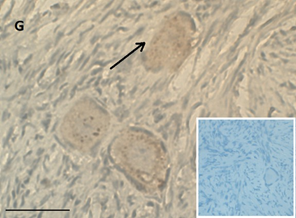
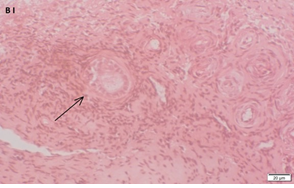
Ovarian section from a 16-year-old girl before therapy (patient no. 26). Note the weak staining intensity in the oocyte & GC for BMP15 (arrow). Original magnification X400. Scale bar=30µm at the left lower corner. The primary antibody used was an anti GDF9B antibody (Santa Cruz Biotechnology, sc-18337). The negative control was prepared by absorbing the primary antibody with its blocking peptide (Santa Cruz Biotechnology, sc-8337P) The negative control is at the bottom right corner. Note the follicle and the exclusive blue hematoxylin staining.
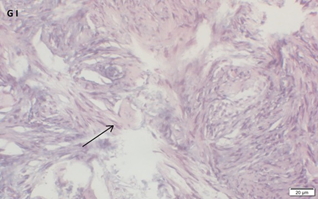
An Hematoxylin and Eosin stained section of the same patient as in 1G. Note the primordial follicle (arrow).
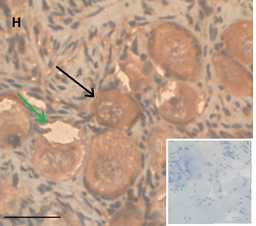
Ovarian section from the same patient as in panel 1A. Note the strong staining intensity for BMP15 in the oocytes and GCs (black arrow) and that some of the follicles were abnormal-crescent shaped (green arrow). Original magnification X400. Scale bar=30µm at the left lower corner. The primary antibody used was a goat polyclonal anti GDF9B antibody (Santa Cruz Biotechnology, sc-18337). The negative control was prepared by absorbing the primary antibody with its blocking peptide (Santa Cruz Biotechnology sc-8337P). The negative control is at the bottom right corner. Note the follicles and the exclusive blue hematoxylin staining.
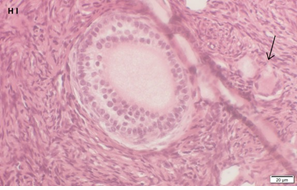
An Hematoxylin and Eosin stained section of the same patient as in 1H. Note the secondary follicle in the center and the primordial follicle (arrow).
Figure 1: IHC staining for the various growth factors.
3.3 Ovarian sources for IF
Ovarian samples were retrieved from 21 girls (Table 1A and 1B). Twelve of the 21 samples were also used for IHC staining, and were proven to contain a large number of follicles, and nine samples were obtained after the IHC part of the study was completed. Fourteen girls were chemotherapy naïve (Table 1A) and seven had been exposed to chemotherapy, including alkylating agents (Table 1B). Nine girls were pre-menarchal including five before chemotherapy and four after chemotherapy, and 12 were post-menarchal, including nine chemotherapy naïve, and three after chemotherapy.
3.4 IF staining results
IF identified 130 follicles from the pre-chemotherapy patients examined: 78 primordial (60%), 38 primary (29%), 14 secondary (11%) and three (9%) atretic or irregularly shaped. In addition, 110 follicles from post-chemotherapy patients were examined: 81 primordial (74%), 22 primary (20%), seven secondary (6%) and 30 (27%) atretic or irregularly shaped. Staining for all three growth factors was identified in the GCs of all pre-antral follicles examined (primordial, primary and secondary). Fluorescent staining was observed in all oocytes and GCs, but intensity was more prominent in the GC. Table 3 summarizes the IF results. Photographs of follicles stained for the growth factors, before and after chemotherapy, are shown in Figure 2 (A-E). Staining for the three growth factors was also observed in atretic or irregularly shaped follicles before or after chemotherapy (Figures 2D and 2E). The nucleus was either unstained or, in most cases, with weak staining intensity for all three factors. Asymmetrical fluorescent staining for all three factors was observed in the cytoplasmic location of the Balbiani body in 46 follicles, regardless of patient age (Figure 2G and 2H): 24 follicles in the pre-chemotherapy group, and 22 follicles in the post-chemotherapy group. However, the follicles were almost exclusively primordial (45/46 follicles, and one primary follicle, P<0.0001 for primordial Vs. primary). There were no significant differences between the pre-chemotherapy and post-chemotherapy groups in the distribution of staining for the three growth factors in the Balbiani body. Balbiani body staining intensity was strong in 30 follicles (65%), medium in 10 follicles (22%), and weak in six follicles (13%). Staining intensity was stronger in the Balbiani body than in the surrounding cytoplasm or GCs (Figure 2G). In the post-chemotherapy group, Balbiani body staining was observed in more follicles from patients that were exposed to alkylating agents than from patients exposed to non-alkylating agents (16 vs. 6 follicles, respectively), but this difference was not statistically significant. Balbiani body staining was also observed in nine of the 33 atretic follicles. The samples from the pre-chemotherapy group had significantly more follicles with strong oocyte staining intensity for AMH and GDF9 than samples from the post-chemotherapy group; predominantly in the oocyte cytoplasm [AMH: (31/130, 24%) vs. (16/110, 14.5%); GDF9: (15/73, 20.5%) vs. (7/45, 15.5%), P<0.0001 for both]. BMP15 cytoplasmic expression intensity was mostly weak or non-existent in both groups. Analysis by pubertal status revealed a significantly higher number of strong AMH primordial follicle cytoplasmic staining intensity in samples from post-menarchal girls compared to pre-menarchal girls (21/43, 49% vs. 8/35, 23%, P=0.02). There were no other differences in growth factor expression that correlated with pubertal status. However, the post-chemotherapy group included only three post-menarchal girls (nos. 41, 43, 44).
|
Follicular staining+intensity |
AMH |
GDF9 |
BMP15 |
|
Before Therapy |
|||
|
Oocyte |
|||
|
None |
12% |
18% |
32% |
|
Partial Full |
58% 31% |
51% 32% |
35% 32% |
|
Balbiani body area |
24 Pf (31%)* |
11 Pf (24.5%)* |
5Pf (11.5%)* |
|
Cytoplasm |
|||
|
None |
11.5% |
18% |
32.5% |
|
Weak |
47.5% |
45% |
49.5% |
|
Medium |
17% |
16.5% |
14% |
|
Strong |
24% |
20.5% |
4% |
|
Nucleus |
|||
|
None |
45% |
41% |
45.5% |
|
Weak |
39% |
38.5% |
41.5% |
|
Medium |
10% |
15% |
9% |
|
Strong |
6% |
5.5% |
4% |
|
Granulosa cells |
|||
|
None |
1.5% |
7% |
1.5% |
|
Weak |
38.5% |
53.5% |
28.5% |
|
Medium |
46% |
34% |
54.5% |
|
Strong |
14% |
5.5% |
15.5% |
|
Stroma |
Scattered |
Scattered |
Scattered |
|
After Therapy |
|||
|
Oocyte |
|||
|
None |
25% |
29% |
22% |
|
Partial |
52% |
56% |
42% |
|
Full |
24% |
16% |
36% |
|
Balbiani body area |
22 Pf (27%)* |
13 Pf (38%)* |
6 Pf (11.3%); 1 primary (6.5%)* |
|
Cytoplasm |
|||
|
None |
23.5% |
29.5% |
22% |
|
Weak |
50% |
37.5% |
52% |
|
Medium |
12% |
17.5% |
8% |
|
Strong |
14.5% |
15.5% |
1.5% |
|
Nucleus |
|||
|
None |
47% |
35.5% |
41% |
|
Weak |
44.5% |
60% |
49.5% |
|
Medium |
7.5% |
4.5% |
9% |
|
Strong |
1% |
1.5% |
|
|
Granulosa cells |
|||
|
None |
1% |
66.5% |
1.5% |
|
Weak |
52.5% |
33.5% |
56% |
|
Weak-medium |
25.5% |
- |
23.5% |
|
Medium |
21% |
- |
19% |
|
Stroma |
Scattered |
Scattered |
Scattered |
*Staining intensity in Balbiani body region was stronger than in the surrounding cytoplasm or granulosa cells
Pf, primordial follicles
Table 3: Ovarian Immunofluorescent Expression of AMH, GDF9 and BMP15 in human ovaries before and after chemotherapy.
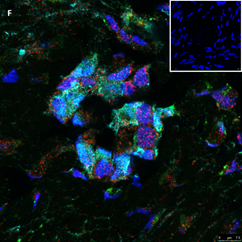
Section of two primary follicles from a 3-year-old girl before chemotherapy (patient no. 3). Note the green GDF9 staining and the red AMH staining in the oocyte and GCs. Scale bar=25µm. The negative control is in the upper right corner. Note the follicles and the exclusive blue DAPI Staining.
Section of a secondary follicle from the same patient as in Figure 2A. Note the red GDF9 staining in the GCs and oocyte (also in the nucleus), the green BMP15 staining mainly in the GCs and the weak cyan AMH staining intensity in the GCs and oocyte cytoplasm. Scale bar=50µm. The negative control is in the upper right corner. Note the exclusive blue DAPI Staining.
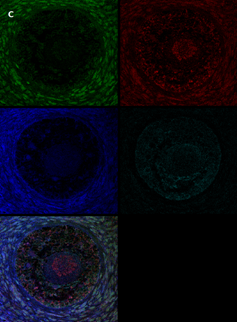
Same section as in panel 2B showing staining for each growth factor alone. Note in the upper left-hand side the green BMP15 staining mainly in the GCs and stroma cells, in the upper right-hand side the red GDF9 staining in the GCs and oocyte (also in the nucleus), in the right-hand side of the middle panel the weak cyan AMH staining intensity in the GCs and oocyte cytoplasm. The blue staining in the middle-left panel is background DAPI. The image in the bottom left hand side is the same merged combination as in panel 2B. Scale bar=50µm
Section of abnormal primary follicle from the same patient as in panel 1C. A Merged image for AMH and BMP15 IF staining. Note the red AMH staining mainly in the GCs. Green BMP15 staining is almost invisible in the merged image. Scale bar=25µm. The negative control is in the upper right corner. Note the follicles and the exclusive blue DAPI Staining.
The same follicle as in panel 2D. Note the green BMP15 staining. Scale bar=25µm
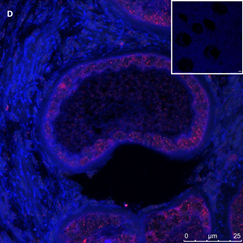
Section of an atretic follicle from a 9-year-old girl after chemotherapy (patient no. 38). Note the abnormal shrunken follicle with the green GDF9 staining, the cyan BMP15 staining mainly in the oocyte and the red AMH staining mainly in the GCs. Scale bar=75µm. The negative control is in the upper right corner. Note the barely visible follicle and the exclusive blue DAPI staining in the background.
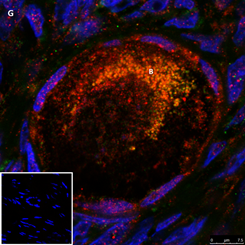
Section of a primordial follicle from a chemotherapy naïve 17-year-old girl (patient no. 29). Note the combined green GDF9 and red AMH staining in the Balbiani (B) body and the red AMH staining in the granulosa cells. Scale bar=75µm. The negative control is in the bottom left corner. Note the follicle and exclusive blue DAPI Staining in the background.
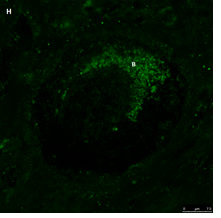
The same primordial follicle as in panel 2G. Note the green GDF9 staining in the Balbiani (B) body. Scale bar=75µm.
Figure 2: IF staining for the various growth factors.
4. Discussion
Our study is the first to show the protein expression of AMH, GDF9, and BMP15 in follicles of pediatric patients after exposure to chemotherapy. All three growth factors were identified by both IHC and IF from primordial stages onwards in oocytes and GCs. Although the number of follicles that positively expressed all three growth factors was higher in the pre-chemotherapy than in the post-chemotherapy group, the difference was not statistically significant. There were no differences in growth factor expression between follicle classes (primordial, primary and secondary). These growth factors were also expressed in atretic follicles in both groups. There were significantly more IHC-stained atretic follicles in post-chemotherapy patients, specifically in GCs; staining intensity was weaker for AMH and GDF9 than for BMP15 (although fewer follicles stained for BMP15). The IF studies made it possible to identify for the first time the expression of these proteins in the Balbiani body (even in atretic follicles in both groups), and in the post-chemotherapy group, even in follicles exposed to alkylating agents. Within the pre-chemotherapy group, IHC cytoplasmic staining for AMH had the strongest intensity at age <8 years, and became weaker thereafter. On IF staining, post-menarchal girls had a significantly higher number of AMH cytoplasmic staining in primordial follicles than pre-menarchal girls. IHC oocyte cytoplasmic staining for all three growth factors seemed fuller before chemotherapy than after. In general, differences between the IHC and IF studies were due either to the higher sensitivity of confocal laser microscopy or the higher number of follicles examined by light microscopy (IHC). The stronger cytoplasmic staining intensity of AMH on IHC study in the younger AMH pre-chemotherapy girls (aged <8 years) than the older girls is in line with the known age-related AMH increase from birth to 8 years [5]. No such difference was noted for GDF9 and BMP15, which are structurally similar to each other and distinct from AMH, which may explain their different staining pattern [38]. However, the number of patients <8 years old was relatively low. Furthermore, there were fewer chemotherapy naive patients <8 years in the IF studies than in the IHC studies, which might account for our failure to identify a similar pattern by IF. Even though tissue from the same patients was used in parallel to identify all three growth factors, IHC yielded similarities only in GC AMH and GDF9 expression but not in BMP15, perhaps because different paraffin block levels were used and they did not contain the same follicles. The pre-chemotherapy group showed significantly stronger IF staining intensity of AMH and GDF9 in the oocyte cytoplasm compared to the post-chemotherapy group; this finding might have been due to the follicles being more viable follicles before chemotherapy. Accordingly, our previous study showed that post-chemotherapy intracellular changes occur also in patients <20 years [3]. We can possibly explain the presence of morphologically normal follicles in all sections studied in the present study by the young age of the patients. Together, the observations in the present study and earlier one [3] might suggest that some of the remaining post-chemotherapy pre-antral follicles have a lower developmental potential. The IHC finding of a higher albeit non- significant number of follicles that positively expressed all three growth factors in the pre-chemotherapy group than the post-chemotherapy group is in agreement with our previous observation of a non-significant post-chemotherapy reduction in follicular number in ovaries pediatric patients [3, 5]. The significantly higher growth factor IHC staining intensity in post-chemotherapy than in pre-chemotherapy atretic follicles, might indicate the presence of a still unknown growth factor follicle-rescue mechanism.
In the present study, we observed IF staining for all three proteins (AMH, GDF9, and BMP15) in the region of the Balbiani body; in most cases the staining intensity was stronger in the Balbiani body than in the surrounding cytoplasm or GCs, possibly because of some, as yet unknown, association of the Balbiani body with growth factors. It is possible that the presence of growth factors in this location is related to selection/elimination of dysfunctional mitochondria from female germline cells [35] at some point in follicle activation. Our finding that more follicles stained in the Balbiani body region after exposure to alkylating agents than to non-alkylating agents might suggest that this location is more resistant to the gonadotoxic effects of alkylating agents than other follicular structures. Previous studies [23, 29] used IHC to examine AMH protein expression in ovaries from human fetuses, girls from pre-puberty to various pubertal stages [29] and normal cycling women [23, 29]. They identified AMH protein expression in follicles only from primary stages onwards, specifically in GCs. In the present study, AMH protein expression was demonstrated in ovaries from young girls by both by IHC and IF, already from primordial stages and not only in the GCs but also in the oocyte cytoplasm and nucleus including the Balbiani body. We were able to differentiate the Balbiani body from the rest of the oocyte cytoplasm by confocal laser microscopy but not by light microscopy. IF staining intensity was stronger in the cytoplasm of primordial follicles from post-menarchal than pre-menarchal girls. Moreover, we identified, for the first time, AMH protein expression in atretic follicles.
Earlier studies from our laboratory reported GDF9 and BMP15 IHM protein expression already from primordial stages in ovaries from pediatric patients before chemotherapy. These proteins were identified: in GCs, oocyte cytoplasm, and nuclei [1, 12]. These results are in agreement with the present study. Others described the expression of both GDF9 and BMP15 in GCs and oocytes of adult women [32, 39]. using various methods including regular IHC, standard IF, and in situ hybridization to identify mRNA transcripts. However, they did not find expression of the proteins in atretic follicles or after chemotherapy. It is noteworthy that one group reported that ovaries of young girls contained large numbers of atretic follicles, regardless of chemotherapy [40]. Indeed, in the present study 17% of the follicles from the chemotherapy-naïve girls were atretic (by IHC). The present study demonstrated post-chemotherapy growth factor expression in all follicular sites in pediatric patients, despite the post-chemotherapy-induced ultracellular follicular changes that were observed by us in an earlier study [3]. The normal growth factor distribution (indicating some degree of follicle viability) together with the high numbers of remaining follicles in ovaries from post-chemotherapy pediatric patients [3, 5], suggest that it is worthwhile to cryopreserve ovarian tissue from pediatric patients even after chemotherapy initiation, including alkylating agent exposure. It will also be interesting to investigate in the future if follicular growth factor expression is similar between pediatric patients and adult women before and after chemotherapy. As for the presence of these growth factors in the location of the Balbiani body, even after exposure to alkylating agents, further studies should be conducted to correlate these growth factors with the numerous intracellular organelles present in this region.
Acknowledgements
The authors are grateful to Ms. Gloria Ganzach, from the editorial board of Rabin Medical Center for the English editing and to Ms. Carmela Felz from the Pathology Institute of Rabin Medical Center for the histological sectioning.
Contributors
- Prag Rosenberg conducted the IF part of the study and wrote most of the manuscript
- Maor conducted the IHC part of the study and wrote parts of the manuscript
- Fisch assisted in designing the study, assisted in the various drafts of the manuscript, the analysis of the results and approved its final version
- Oron provided some of the ovarian samples, assisted in the histological preparation and reviewed the final draft of the manuscript
- Ash recruited most of the patients, helped in writing the manuscript and critically proofed the final manuscript
- Anuka assisted with the IF studies and advised regarding IF staining on paraffin sections. He critically proofed the final manuscript.
- Yaniv recruited most of the patients, helped in writing the manuscript and critically proofed the final manuscript
- Freud performed the surgery on the patients, wrote certain parts of the manuscript and critically proofed the final manuscript
- Ben Arush referred some of the patients to the study and assisted with the manuscript's drafting
- Mhameed assisted with the IF studies and reviewed the final draft of the manuscript
- Ben-Haroush provided some of the ovarian samples and reviewed the final draft of the manuscript
- Ben-Aharon recruited most of the patients, helped to design the study, wrote certain parts of the manuscript and critically proofed the final manuscript
- Shufaro assisted in designing the study, assisted in the various drafts of the manuscript, the analysis of the results and approved its final version
- Abir designed the study and collected the data, assisted in IHC and IF studies and viewed the sections, conducted most of the statistical analysis and wrote and revised the manuscript.
References
- Bath LE, Wallace WHB, Critchley HOD. Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG 109 (2002): 107-114.
- Feigin E, Freud E, Fisch B, et al. Fertility preservation in female adolescents with malignancies. In Moorland MT. Cancer in female adolescents. Hauppauge, Nova Science Publishers NY (2008): 103-38.
- Abir R, Ben-Haroush A, Felz C, et al. Selection of patients before and after anticancer treatment for ovarian cryopreservation. Hum Reprod 23 (2008): 869-877.
- Fisch B, Abir R. Female fertility preservation: past, present and future.Reproduction 156 (2018): F11-F27.
- Abir R, Ben-Aharon I, Garor R, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in pediatric female cancer patients before and after cancer therapy. Hum Reprod 31 (2016): 750-762.
- Donnez J, Dolmans MM. Fertility Preservation in Women. New England Journal of Medicine 377 (2017): 1657-1665.
- Demeestere I, Simon P, Dedeken L, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod 30 (2015): 2107-2109.
- Matthews SJ, Picton H, Ernst E. Successful Pregnancy in a Woman Previously Suffering From β-Thalassemia Following Transplantation of Ovarian Tissue Cryopreserved Before Puberty. Minerva Ginecol 70 (2018): 432-435.
- Familiari G, Caggiati A, Nottola SA, et al. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum Reprod 8 (1993): 2080-2087.
- La Marca A, Sighinolfi G, Radi D, et al. Anti Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 16 (2010): 113-130.
- Oron G, Fisch B, Ao A, et al. Expression of growth-differentiating factor 9 and its type 1 receptor in human ovaries. Reproductive Biomedicine Online 21 (2010): 109-117.
- Margulis S, Abir R, Felz C, et al. Bone morphogenetic protein 15 expression in human ovaries from fetuses, girls, and women. Fertil Steril 92 (2009): 1666-1673.
- Durlinger AL, Kramer P, Karels B, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 140 (1999): 5789-5796.
- Dong J, Albertini DF, Nishimori K, et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383 (1996): 531-535.
- Yan C, Wang P, DeMayo J, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 15 (2001): 854-866.
- Shimasaki S, Moore RK, Otsuka F, et al. The bone morphogenetic protein system in mammalian reproduction. Endocrine Reviews 25 (2004): 72-101.
- Fabre S, Pierre A, Mulsant P, et al. Regulation of ovulation rate in mammals: contribution of sheep genetic models. Reprod Biol Endocrinol 4 (2006): 20.
- Kovanci E, Rohozinski J, Simpson JL, et al. Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil Steril 87 (2007): 143-146.
- McMahon HE, Hashimoto O, Mellon PL, et al. Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice. Endocrinology 149 (2008): 2807-2815.
- Abir R, Fisch B. Invited commentary: a single nucleotide polymorphism in BMP15 is associated with high response to controlled ovarian hyperstimulation. Reprod Biomed Online 23 (2011): 77-80.
- Kedem A, Fisch B, Garor R, et al. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J Clin Endocrinol Metab 96 (2011): E1246-E1254.
- Alvaro Mercadal B, Imbert R, Demeestere I, et al. AMH mutations with reduced in vitro bioactivity are related to premature ovarian insufficiency. Hum Reprod 30 (2015): 1196-1202.
- Weenen C, Laven JS, Von Bergh AR, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10 (2004): 77-83.
- Iwase A, Osuka S, Goto M, et al. Clinical application of serum anti-Müllerian hormone as an ovarian reserve marker: A review of recent studies. J Obstet Gynaecol Res 44 (2018): 998-1006.
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction 124 (2002): 601-609.
- Schmidt KLT, Kryger-Baggesen N, Byskov AG, et al. Anti-Mullerian hormone initiates growth of human primordial follicles in vitro. Mol Cell Endocrinol 234 (2004): 87-93.
- Carlsson IB, Scott JE, Visser JA, et al. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod 21 (2006): 2223-2227.
- Xu J, Bishop CV, Lawson MS, et al. Anti-Müllerian hormone promotes pre-antral follicle growth, but inhibits antral follicle maturation and dominant follicle selection in primates. Hum Reprod 31 (2016): 1522-1530.
- Rajpert-De Meyts E, Jørgensen N, Graem N, et al. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab 84 (1999): 3836-3844.
- Hreinsson JG, Scott JE, Rasmussen C, et al. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab 87 (2002): 316-321.
- Teixeira Filho FL, Baracat EC, Lee TH, et al. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab 87 (2002): 1337-1344.
- Wei LN, Huang R, Li LL, et al. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J Assist Reprod Genet 31 (2014): 1483-1490.
- Kloc M, Bilinski S, Etkin LD. The Balbiani body and germ cell determinants: 150 years later. Curr Top Dev Biol 59 (2004): 1-36.
- De Smedt V, Szollosi D, Kloc M. The Balbiani body: asymmetry in the mammalian oocyte. Genesis 26 (2000): 208-212.
- Bilinski SM, Kloc M, Tworzydlo W. Selection of mitochondria in female germline cells: Is Balbiani body implicated in this process? J Assist Reprod Genet 34 (2017): 1405-1412.
- Anuka E, Yivgi-Ohana N, Eimerl S, et al. Infarct-induced steroidogenic acute regulatory protein: a survival role in cardiac fibroblasts. Mol Endocrinol 27 (2013): 1502-1517.
- Hewitt SM, Baskin DG, Frevert CW, et al. Controls for immunohistochemistry: the Histochemical Society's standards of practice for validation of immunohistochemical assays. Journal of Histochemistry and Cytochemistry 62 (2014): 693-697.
- De Castro FC, Cruz MH, Leal CL. Role of Growth Differentiation Factor 9 and Bone Morphogenetic Protein 15 in ovarian function and their importance in mammalian female infertility – a review. Asian-Australas J Anim Sci 29 (2016): 1065-1074.
- Chen Y, Zhao S, Qiao J, et al. Expression of bone morphogenetic protein-15 in human oocyte and cumulus granulosa cells primed with recombinant follicle-stimulating hormone followed by human chorionic gonadotropin. Fertil. Steril 92 (2009): 2045-2046.
- Anderson RA, McLaughlin M, Wallace WH, et al. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod 29 (2014): 97-106.
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 17 (1996): 121-155.
