Oral Anticoagulants in Low-Risk Atrial Fibrillation Patients: A Population-Based Study
Article Information
Gentian Denas1, Nicola Gennaro2, Eliana Ferroni2, Ugo Fedeli2, Giacomo Zoppellaro3, Maria Chiara Corti2, and Vittorio Pengo1*
1Cardiology Clinic, Department of Cardiac, Thoracic and Vascular Sciences, University of Padova, Padua, Italy
2Epidemiological Department (SER), Azienda Zero, Veneto Region, Padua, Italy
3Cardiology, Azienda ULSS 3 Serenissima, Ospedale Civile, Venezia, Italy
*Corresponding author: Vittorio Pengo, Cardiology Clinic, Department of Cardiac, Thoracic and Vascular Sciences, University of Padova, Padua, Italy
Received: 03 January 2020; Accepted: 11 January 2020; Published: 03 February 2020
Citation: Gentian Denas, Nicola Gennaro, Eliana Ferroni, Ugo Fedeli, Giacomo Zoppellaro, Maria Chiara Corti, and Vittorio Pengo. Oral Anticoagulants in Low-Risk Atrial Fibrillation Patients: A Population-Based Study. Cardiology and Cardiovascular Medicine 4 (2020): 066-075.
View / Download Pdf Share at FacebookAbstract
Aim: The use of oral anticoagulant drugs in patients with atrial fibrillation and one non-gender related risk factors is challenging. We compared the efficacy and safety of DOACs vs VKAs in low risk patients.
Methods: We performed a population-based retrospective cohort study in anticoagulation-naïve atrial fibrillation patients. The cohort was identified, characterized and followed-up using data from administrative claims, drug prescriptions archive, and regional inpatient and discharge register. Event-rates were assessed using as treated analysis. Hazard ratios (HR) of stroke and major bleeding were estimated by Cox regression analysis.
Results: Overall, we identified 1829 patients treated with DOACs and 6083 patients treated with VKAs, that accumulated 2097 and 4681 person-years of follow up, respectively. Half of patients were in the 65-75 age group, while almost 38% were female. Stroke rates were lower with DOACs as compared to VKAs: 0.14% person-years versus 0.28% person-years (HR 0.50, 95%CI 0.14–1.74). Major bleeding (0.81% person-years versus 1.09% person-years (HR 0.80, 95%CI 0.46–1.40)) and intracranial hemorrhage (0.33% person-years versus 0.42% person-years (HR 0.85, 95%CI 0.36–2.04) were also lower with DOACs. Mortality rate with DOACs was 1.2% person-years and 1.1 person-years with VKA (HR 1.21, 95%CI 0.74-1.96) mostly driven by death from cancer in the DOACs group.
Conclusions: In low risk patients with atrial fibrillation, there is a benefit (although non-significant) with DOACs as compared to VKAs. Other studies are required to directly test this finding.
Keywords
Atrial fibrillation; Direct oral anticoagulants; Vitamin K antagonists; Low stroke risk; CHA2DS2VASc
Article Details
Abbreviations
DOACs - Direct Oral Anticoagulants
VKAs - Vitamin K Antagonists
AF - Atrial Fibrillation
OACs - Oral Anticoagulants
IS - Ischemic Stroke
ICH - Intracranial Hemorrhage
HR - Hazard Ratios
CI - Confidence Intervals
Introduction
The CHA2DS2VASc score has become the standard for assessing the risk of stroke in atrial fibrillation (AF) [1]. A score of 0 in males (or 1 in females) defines a very low risk of stroke that advises against the use of anticoagulation. On the other hand, a score of ≥2 in male and ≥3 in female patients defines an expected net clinical benefit in favor of anticoagulation [2]. The decision on whether to treat patients with a CHA2DS2VASc score of 1 in male or 2 in female, i.e. those with one non-gender related risk factor (NGR-RF), is subject to debate [2-8]. In this patient group, observational studies have reported variable rates of ischemic stroke without anticoagulation [6-9], making it difficult to definitely establish a clear benefit of treatment. This uncertainty is reflected by the guidelines [10,11] in which the use of anticoagulants based on an individualized weighting of risk. There are some reports showing that benefits of anticoagulation with vitamin K antagonists (VKAs) in patients with one NGR-RF may not exceed the risks of bleeding [3,7,8,11]. Direct oral anticoagulants (DOACs) have shown a better safety profile as compared to VKAs in registration trials and real-life cohorts. However, the benefit obtained in high-risk populations (CHA2DS2 score of 2 or more) might not translate to low-risk patients [13].
In this cohort of patients with one NGR-RF, we evaluated the rate of stroke major bleeding and death among low stroke risk patients treated with DOACs as compared to well-managed VKA therapy.
Methods
Study setting
We performed a population-based retrospective analysis on linked administrative claims in the Veneto Region, Italy, using the drug prescriptions archive (ATC codes), the regional inpatients register (ICD9-CM coded discharge diagnoses), the database of residents registered in the regional health system, the archive of co-payment exemptions to identify anticoagulation naive patients with non-valvular atrial fibrillation. Detailed identification of naive patients with atrial fibrillation and their characteristics has been previously published [14]. Patient recruitment and follow up extended from July 1, 2013 to December 31, 2017. All patients had at least 3 months of follow up. From this cohort, we extracted the population of male patients with a CHA2DS2VASc score of 1 and female patients with a CHA2DS2VASc of 2. The first prescription of oral anticoagulants (OACs), or index date, identified the date of enrolment in the cohort. We excluded from enrolment individuals with any dispensed prescription of OACs in the 12 months preceding the index date.
Patient exposure to anticoagulation was calculated on an as treated basis. Patients exited the study in the absence of a new prescription by the end of a 60-day period (grace time) from the last identified index medication fill, occurrence of an endpoint or the end of follow up, whichever came first. VKA dosage was calculated using defined daily doses (DDD) counting one DDD per day and distributing all available DDDs to days of follow-up (including the days covered by the last prescription).
Detailed information including drug prescription, relevant comorbidities for risk score calculation (CHA2DS2VASc and HASBED: a modified score that did not include labile INR) were available for all patients in the registry.
Endpoint definition
Study endpoints were ischemic stroke (IS), death from any cause, and major bleeding including intracranial hemorrhage (ICH), gastrointestinal bleeding, genitourinary bleeding, and bleeding from other sites identified according to ICD-9CM codes. Major bleeding was identified using the Cunningham algorithm for automated database definition of serious bleeding related to oral anticoagulant use [15]. We excluded intracranial hemorrhage associated with a concomitant discharge diagnosis of major trauma and events not leading to hospitalization or those that occurred as a complication of a hospitalization for another problem. To avoid overestimation of events, a 30-day blanking period was set for ischemic stroke; events occurring in the blanking period were excluded from the final analysis [6]. For the other endpoints, we started counting the days at risk from the date of endpoint occurrence.
The study was exempt from institutional review board approval because there was no direct patient involvement and we used pre-existing deidentified data.
Statistical analysis
Baseline characteristics are presented and compared as appropriate. The rate of events for the assessed endpoints is expressed as number of events per 100 patient-years. A time-to-event analysis was adopted to measure the risk of study endpoints from the initial prescription until the occurrence of ischemic stroke, major bleeding, death, switch to a different anticoagulant drug, discontinuation of the index anticoagulant drug, or the end of follow-up, whichever came first. Cox regression was used to compare event rates between groups with results expressed as hazard ratios (HR) with 95% confidence intervals (CI). Possible predictors of major bleeding and intracranial bleeding were assessed in a Cox multivariate analysis.
Results
Of the 7912 anticoagulation-naive patients with one NGR-RF, 1829 received DOACs and 6083 received VKAs. Baseline characteristics are presented in Table 1.
|
Clinical Characteristic, n (%) |
DOACs (n=1829) |
VKAs (n=6083) |
|
Age 65-74 y |
958 (52.4) |
3245 (53.3) |
|
Female |
740 (40.5) |
2236 (36.8) |
|
Congestive heart failure |
7 (0.4) |
33 (0.5) |
|
Hypertension |
795 (43.5) |
2619 (43.1) |
|
Diabetes mellitus |
64 (3.5) |
161 (2.7) |
|
Vascular disease/coronary artery disease |
4 (0.2) |
30 (0.5) |
|
Abnormal Renal/Liver function |
21 (1.1) |
163 (2.7) |
|
History of bleeding |
8 (0.4) |
33 (0.5) |
|
Medications predisposing to bleeding* |
772 (42.2) |
2362 (38.8) |
|
Cardioversion |
115 (6.3) |
938 (15.4) |
|
CHA2DS2VASc |
||
|
1 |
1089 (59.5) |
3847 (63.3) |
|
2 |
740 (40.5) |
2236 (36.7) |
|
HASBED |
||
|
0-2 |
1806 (98.7) |
6008 (98.8) |
|
≥3 |
23 (1.3) |
75 (1.2) |
Table 1: Characteristics of the cohort
DOACs – direct oral anticoagulants; VKAs – vitamin K antagonists
* more than 3 prescriptions of antiplatelet or nonsteroidal anti-inflammatory drugs.
There were no major differences between the groups.
Among the CHA2DS2VASc items, age and hypertension were the most represented risk factors. Noteworthy, patients had also a low bleeding risk as assessed by the HASBED score.
Follow up extended for 6778 person-years; 2097 person-years in the DOAC treated individuals and 4681 person-years in the VKA treated individuals.
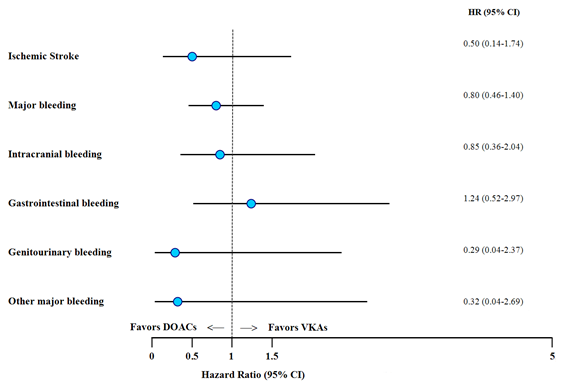
The overall risk of events was lower with DOACs, except for GI bleeding (Figure 1). The rate of ischemic stroke was 0.24% person-years in the entire cohort and was lower among the DOAC as compared to VKA treated patients (0.14% person-years vs. 0.28% person-years; HR 0.50, 95%CI 0.14–1.74).
The overall major bleeding rate was 1% person-years. The rate of major bleeding was lower in the DOAC group as compared to VKAs (0.81% person-years vs 1.09% person-years; HR 0.80, 95%CI 0.46-1.40). ICH was lower in the DOACs group (0.33% person-years vs 0.42% person-years; HR 0.85, 95%CI 0.36-2.04), while there was a trend towards higher GI bleeding rates with DOACs (0.38% person-years vs 0.32% person-years; HR 1.24, 95%CI 0.52-2.97). Genitourinary bleeding and other major bleedings occurred at a lower rate in the DOAC group.
As shown in Table 2, there were no predictors of major or ICH bleeding among the plausible risk factors (female gender, age > 65 years, hypertension and medications predisposing to bleeding).
|
Event rate |
HR (95% CI) |
p-value |
|
|
Major bleeding |
|||
|
DOAC |
0,81 |
0.81 (0.46 - 1.40) |
p=0.452 |
|
VKA |
1,08 |
||
|
Gender, F |
0,96 |
0.93 (0.56 – 1.53) |
p=0.780 |
|
Age >/= 65 |
1,09 |
1.21 (0.75 – 1.96) |
p=0.429 |
|
Hypertension |
0,95 |
0.91 (0.56 - 1.48) |
p= 0.722 |
|
Diabetes |
0,47 |
0.47 (0.06 – 3.43) |
p=0.463 |
|
Renal disease |
1,64 |
1.50 (0.20 – 10.8) |
p= 0.686 |
|
History or predisposition to bleeding |
1,18 |
1.39 (0.86 – 2.24) |
p= 0.594 |
|
Medications predisposing to bleeding |
1,19 |
1.42 (0.88 - 2.28) |
p=0.146 |
|
HASBED >/= 2 ** |
1,21 |
1.38 (0.83 – 2.27) |
p= 0.605 |
|
Intracranial bleeding (ICH)* |
|||
|
NOAC |
0,33 |
0.85 (0.36 - 2.04) |
p=0.729 |
|
VKA |
0,42 |
||
|
Gender, F |
0,4 |
1.01 (0.46 - 2.21) |
p=0.975 |
|
Age >/= 65 |
0,5 |
1.80 (0.81-4.02) |
p=0.147 |
|
Hypertension |
0,3 |
0.65 (0.29-1.45) |
p=0.298 |
|
Medications predisposing to bleeding |
0,49 |
1.53 (0.72-3.26) |
p=0.267 |
|
HASBED >/= 2 ** |
0,5 |
1.48 (0.67-3.24) |
p=0.351 |
Table 2: Multivariate analysis assessing the predictors of major bleeding and ICH.
* No ICH occurred in patients with Diabetes and Renal disease; ** HASBED>/= 2 was used because there were no events in patients with HASBED >/= 3.
Overall, mortality rates with DOACs and VKAs were 1.2% per person years and 1.1% per person years, respectively (HR 1.21, 95%CI 0.74-1.96). Death from cancer were 17/26 (65%) in the DOACs group and 28/59 (47%) in the VKAs group (Table 3, p=0.1). On the other hand, death from cardiovascular causes was 3/26 (11%) in the DOACs group and 15/59 (25%) in the VKAs group.
|
|
DOACs n=26 |
VKAs n=59 |
|
Cancer, n (%) |
17 (65) |
28 (47) |
|
Cardiovascular death, n (%) |
3 (11) |
15 (25) |
|
Ischemic heart disease |
1 |
3 |
|
Cerebrovascular |
1 |
3 |
|
Other cardiovascular |
1 |
9 |
|
Other, n (%) |
6 (23) |
16 (27) |
Table 3: Causes of death in the DOAC and VKA treated patients.
DOACs – direct oral anticoagulants; VKAs – vitamin K antagonists
Discussion
The question on whether to treat patients with one NGR-RF with oral anticoagulants is still subject to debate [2-8]. Despite uncertainty, we found a large number of patients with one NGR-RF receiving oral anticoagulation. Most patients possibly received anticoagulation in alignment with the European guideline recommendations [16] for the management of AF for patients with 1 NGR-RF (Class IIaB). The present study was prompted by a previous prospective study [7], in which we found a concerning incidence of major bleeding and ICH with warfarin in patients with one NGR-RF. In fact, the introduction of DOACs might have been beneficial in this setting. It has been shown that the absolute advantage of VKA-anticoagulation increases with increasing thromboembolic risk [13]. DOACs have a greater net clinical benefit over VKAs when there is a high risk of both ischemic stroke and intracranial hemorrhage [17]. How much this advantage flattens in low risk patients is not clear. Moreover, at variance with most patients with AF, these are younger with a good renal function which might be a concern for the efficacy of DOACs. In contrast, we found a tendency towards a reduction in ischemic stroke rates with DOACs as compared to VKAs. Although statistical significance was not reached, our findings show that patients treated with DOACs have a 50% reduction in stroke rates. Same is true for safety as the absolute reduction of major bleeding with DOACs was 20% and that of ICH was 15%. These findings are relevant because, despite their extensive use, DOACs have not been specifically tested in randomized trials for efficacy in patients with one NGR-RF.
Despite the overall favorable safety profile of DOACs versus VKAs, we found confirmation that gastrointestinal bleeding is increased (24%) with DOACs even in this younger low-risk population.
Major bleeding is a major concern when treating with anticoagulation in general and particularly in patients at low risk of stroke. Bleeding scores are generally not solid in predicting major bleeding, particularly cerebral bleeding. In this study, possible risk factors for bleeding were tested in multivariate analysis and none of them resulted a significant predictor.
Mortality rate was slightly higher in the group of patients treated with DOACs. However, mortality for cancer but not that attributable to cardiovascular diseases was prevalent in the group of patients treated with DOACs. This observation led to the hypothesis that anti-cancer drugs (i.e. anthracyclines) cause heart problems including anticancer therapy-induced AF [18].
With our data, we cannot answer the question on whether anticoagulation is warranted in these patients; this should be possibly considered in case-by-case basis, using other tools [19,20] and considering other unaccounted risk factors like biomarkers, patterns of atrial fibrillation, and atrial function [20-23]. However, when anticoagulation is deemed necessary, using DOACs instead of VKAs offers some benefit even in patients with one NGR-RF.
Limitations of our study are the retrospective nature of the design and data collection through routine health records without direct patient involvement. On the other hand, strengths of this study are the involvement of a high number of patients and the accuracy of cohort selection and endpoint identification.
Conclusion
In conclusion, whenever anticoagulation is indicated in low-risk patients with atrial fibrillation, DOACs provide an overall noteworthy benefit over VKAs.
Acknowledgements
Funding: This work was supported by an unconditional grant of the Veneto Region, Italy [BUR n. 27 of 20/03/2015].
Declarations of interest
All the authors have no conflict of interest to declare
References
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137 (2010): 263-72.
- Huisman MV. Patients with atrial fibrillation and a CHA2DS2-VASc score of 1: are they at low or high stroke risk? J Am Coll Cardiol 65 (2015): 1395-1397.
- Singer DE, Ezekowitz MD. Adding rigor to stroke risk prediction in atrial fibrillation. J Am Coll Cardiol 65 (2015): 233-235.
- Coppens M, Eikelboom JW, Hart RG, et al. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J 34 (2013): 170-176.
- Lip GY, Skjoth F, Rasmussen LH, Nielsen PB, Larsen TB. Net Clinical Benefit for Oral Anticoagulation, Aspirin, or No Therapy in Nonvalvular Atrial Fibrillation Patients With 1 Additional Risk Factor of the CHA2DS2-VASc Score (Beyond Sex). J Am Coll Cardiol 66 (2015): 488-490.
- Friberg L, Skeppholm M, Terent A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J Am Coll Cardiol 65 (2015): 225-232.
- Denas G, Zoppellaro G, Padayattil Jose S, et al. Warfarin prescription in patients with nonvalvular atrial fibrillation and one non-gender-related risk factor (CHA2 DS2 VASc 1 or 2): A treatment dilemma. Cardiovasc Ther 36 (2018).
- Allan V, Banerjee A, Shah AD, et al. Net clinical benefit of warfarin in individuals with atrial fibrillation across stroke risk and across primary and secondary care. Heart 103 (2017): 210-218.
- Lip GY, Skjoth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol 65 (2015): 1385-1394.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37 (2016): 2893-2962.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019.
- Quinn GR, Severdija ON, Chang Y, Singer DE. Wide Variation in Reported Rates of Stroke Across Cohorts of Patients With Atrial Fibrillation. Circulation 135 (2017): 208-219.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 151 (2009): 297-305.
- Denas G, Gennaro N, Ferroni E, et al. Effectiveness and safety of oral anticoagulation with non-vitamin K antagonists compared to well-managed vitamin K antagonists in naive patients with non-valvular atrial fibrillation: Propensity score matched cohort study. Int J Cardiol 249 (2017): 198-203.
- Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf 20 (2011): 560-566.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 18 (2016): 1609-1678.
- Lip GY, Al-Khatib SM, Cosio FG, et al. Contemporary management of atrial fibrillation: what can clinical registries tell us about stroke prevention and current therapeutic approaches? J Am Heart Assoc 3 (2014).
- Yang X, Li X, Yuan M, et al. Anticancer Therapy-Induced Atrial Fibrillation: Electrophysiology and Related Mechanisms. Front Pharmacol 9 (2018): 1058.
- Fox KAA, Lucas JE, Pieper KS, et al. Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open 7 (2017): e017157.
- Hijazi Z, Lindback J, Alexander JH, et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 37 (2016): 1582-1590.
- Leung M, van Rosendael PJ, Abou R, et al. Left atrial function to identify patients with atrial fibrillation at high risk of stroke: new insights from a large registry. Eur Heart J 39 (2018): 1416-1425.
- Vanassche T, Lauw MN, Eikelboom JW, et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J 36 (2015): 281a-287a.
- Boriani G, Botto GL, Padeletti L, et al. Improving stroke risk stratification using the CHADS2 and CHA2DS2-VASc risk scores in patients with paroxysmal atrial fibrillation by continuous arrhythmia burden monitoring. Stroke 42 (2011): 1768-1770.