Mandibular First Premolar with Three Canals and Periodontalendodontic Lesion: Case Report
Article Information
Khalaf A Alwasi1, Saad M Alshahrani2, Khaled M El-Shewahy3, Tarek Ezzeldin4*
1Endodontist, Head of Endodontics, Department and Program Director of Saudi Board of Endodontics, Dammam Medical Complex, SaudiArabia
2Endodontist, Chief of the dental department King Abdul Aziz Armed Forces Hospital, SaudiArabia
3Periodontist, King Abdul Aziz Armed Forces Hospital, SaudiArabia
4Pediatric Dentist, Program Director of the Complete Saudi Board of Pediatric Dentistry in E1 Cluster at Dammam Medical Complex, SaudiArabia
*Corresponding author: Khalaf A Alwasi, Consultant & Head of Endodontics Department, Program Director
of Saudi Board of Endodontics, Dammam Medical Complex, SaudiArabia
Received: 20 May 2021; Accepted: 27 May 2021; Published: 31 August 2021
Citation: Khalaf A Alwasi, Saad M Alshahrani, Khaled M El-Shewahy, Tarek Ezzeldin. Mandibular First Premolar with Three Canals and Periodontalendodontic Lesion: Case Report. Dental Research and Oral Health 4 (2021): 049-058.
View / Download Pdf Share at FacebookAbstract
This clinical report presents and describes both the endodontic and periodontal treatment of a mandibular first premolar exhibiting three root canals presented with perio-endo infection. Mandibular first premolars usually have a single root and a single root canal. The incidence of three root canals in this tooth is quite rare. Knowledge of root canal morphology, proper clinical examination, correct radiographic interpretation, using dental operating microscope, and careful tactile examination of the root canals are important in detecting the presence of the multiple canals and in treating the endodontic and periodontal lesions.
Keywords
Periodontal, Root canal anatomy, Mandibular premolar
Periodontal articles; Root canal anatomy articles; Mandibular premolar articles
Periodontal articles Periodontal Research articles Periodontal review articles Periodontal PubMed articles Periodontal PubMed Central articles Periodontal 2023 articles Periodontal 2024 articles Periodontal Scopus articles Periodontal impact factor journals Periodontal Scopus journals Periodontal PubMed journals Periodontal medical journals Periodontal free journals Periodontal best journals Periodontal top journals Periodontal free medical journals Periodontal famous journals Periodontal Google Scholar indexed journals Root canal anatomy articles Root canal anatomy Research articles Root canal anatomy review articles Root canal anatomy PubMed articles Root canal anatomy PubMed Central articles Root canal anatomy 2023 articles Root canal anatomy 2024 articles Root canal anatomy Scopus articles Root canal anatomy impact factor journals Root canal anatomy Scopus journals Root canal anatomy PubMed journals Root canal anatomy medical journals Root canal anatomy free journals Root canal anatomy best journals Root canal anatomy top journals Root canal anatomy free medical journals Root canal anatomy famous journals Root canal anatomy Google Scholar indexed journals Mandibular premolar articles Mandibular premolar Research articles Mandibular premolar review articles Mandibular premolar PubMed articles Mandibular premolar PubMed Central articles Mandibular premolar 2023 articles Mandibular premolar 2024 articles Mandibular premolar Scopus articles Mandibular premolar impact factor journals Mandibular premolar Scopus journals Mandibular premolar PubMed journals Mandibular premolar medical journals Mandibular premolar free journals Mandibular premolar best journals Mandibular premolar top journals Mandibular premolar free medical journals Mandibular premolar famous journals Mandibular premolar Google Scholar indexed journals endodontic articles endodontic Research articles endodontic review articles endodontic PubMed articles endodontic PubMed Central articles endodontic 2023 articles endodontic 2024 articles endodontic Scopus articles endodontic impact factor journals endodontic Scopus journals endodontic PubMed journals endodontic medical journals endodontic free journals endodontic best journals endodontic top journals endodontic free medical journals endodontic famous journals endodontic Google Scholar indexed journals root canal articles root canal Research articles root canal review articles root canal PubMed articles root canal PubMed Central articles root canal 2023 articles root canal 2024 articles root canal Scopus articles root canal impact factor journals root canal Scopus journals root canal PubMed journals root canal medical journals root canal free journals root canal best journals root canal top journals root canal free medical journals root canal famous journals root canal Google Scholar indexed journals premolars articles premolars Research articles premolars review articles premolars PubMed articles premolars PubMed Central articles premolars 2023 articles premolars 2024 articles premolars Scopus articles premolars impact factor journals premolars Scopus journals premolars PubMed journals premolars medical journals premolars free journals premolars best journals premolars top journals premolars free medical journals premolars famous journals premolars Google Scholar indexed journals
Article Details
1. Introduction
Successful endodontic treatment can be achievable with accurate diagnosis & treatment planning, along with having the knowledge of root canal morphology and its frequent variations [1]. It is generally recognized that the incomplete shaping and cleaning of root canals will lead to endodontic failure [2,3]. Amongst all the teeth, Slowey reported that mandibular premolars are the most difficult teeth to treat endodontically due to wide variation in root canal morphology [4]. The internal anatomy of a canal system may demonstrate fins, isthmuses, accessory canals, or diverse canal shapes. Ingle reported that root canals in mandibular first premolars as ovoid at the cervical level, round or ovoid at the mid-root, and round in the apical level. Mueller described the shape of the canals were usually quite round and conical, but inclined to be ribbon- like in the cervical third of the root [5,6]. Mandibular first premolars are known to have a single canal. A thorough understanding of root canal morphology is required for achieving high levels of success in endodontic treatment. Weine categorized the root canal system into four basic types [7]. Vertucci found numerous complex canal systems and identified eight pulp canal configurations and he described five different types of canal configuration for mandibular first premolar [8]. Pineda and Kuttler found 0.9% three root canals in mandibular first premolars radiographically [9].Vertucci using a transparent method found 25.5% of 400 mandibular premolars had two apical openings and 0.5% of the teeth had three apical openings [10]. On the other hand Walker reported 34% southern Chinese had two canals and 2% had three canals radiographically [11]. The fact that both periodontium and dental pulp are anatomically interrelated by virtue of apical foramen and lateral canals creates pathways of exchange of noxious agents between the two tissues [12]. Moreover resorption processes of the root surface, root perforation during endodontic treatment and opening of the dentinal tubules during periodontal treatment establish, yet ,another pathway across the body of the tooth structure [13]. A classification of these relationships was proposed by Simon et al. into Primary endodontic lesion, Primary periodontal lesion, Primary endodontic lesion with secondary periodontal involvement, Primary periodontal lesion with secondary endodontic involvement and True combined lesion [14]. The inflammatory lesions in the periodontal tissue induced and maintained by root canal infection often have a limited extension around the root apex, moreover, pulpal tissue may be significantly inflamed and yet exert little or no effect on the periodontium as long as the pulp remains vital [15,16]. Necrosis of the pulp, however, can result in loss of the periodontal support to the extent that apical marginal communication emerges especially if there is existing periodontal disease [17]. In accordance it seems that periodontal rarely jeopardizes the vital pulp function and retrograde pulpits through periodontal ligament rarely occurs [18]. Neither irreversible pulpits nor pulp necrosis had been reported in histologic studies of teeth extracted because of sever periodontitis [19]. However at the terminal stages, when the plaque microbiota has reached the main apical foramina, pulpal necrosis may result [20]. Occasionally endodontic and periodontal lesions may affect the same tooth and the two lesions may be completely separated or in few situations there is no obvious demarcation between them appearing clinically and radiographically as one lesion (combined lesion defect) [21]. A key consideration in diagnosis and treatment of cases with simultaneous periodontal and pulpal lesions is the extent to which the periodontal lesion contributes to the bone loss [17]. While the part of the lesion sustained by root infection can usually resolve after proper endodontic treatment, the periodontal component is a more difficult problem with little or no regeneration expected after the periodontal treatment [12]. This suggests that the if the majority of bone loss is due to periodontitis, regardless to the predictability of the endodontic therapy, the tooth may have a questionable, poor or even hopeless prognosis [22]. Therefore the proposed treatment strategy should be first to focus on the pulpal infection with debridement and disinfection of the root canal system. After successful endodontic treatment the residual periodontal lesion can be more predictably managed using a varity of treatment options including scaling, root planning or periodontal surgical modalities [23]. This case report describes both endodontic and periodontal treatment performed on a first mandibular premolar with three root canals, two buccal and one lingual, situated in one root, diagnosed with primary periodontal disease with secondary endodontic involvement (Class IV Simon classification), and exacerbation of acute periapical abscess.
2. Case Report
A 39 year old Saudi male with noncontributory medical history was referred to the clinics of the Saudi Board in Endodontics at King Abdul Aziz Armed Force Hospital in Dhahran for evaluation and root canal therapy of a mandibular first premolar. The patient presented with extra oral facial swelling at the right lower mandibular premolar- molar area started three days ago with intermittent throbbing pain started from four months. Clinical intraoral examination revealed diffuse swelling related to tooth #44 with tenderness to percussion and pus oozing on palpation (figure 1), there was also painful enlarged submandibular lymphadenopathy. Vitality tests using Cold test with ROEKO Endo Frost (Coltène/ Whaledent/ Germany), and electric pulp tester using Kerr Vitality Scanner 2006 (SybronEndo, Orange, CA) revealed negative response. Periodontal examination using William's graduated probe revealed the presence of deep pockets 12, 9, 11, 10, 8 and 12mm with attachment loss of 9, 6, 12, 11, 10 and 9 mm at MB, B, DB, DL, L, and ML respectively, and pus oozing on probing (Figure 1). Grade III mobility according to Nyman and Lindhe classification was noticed [24]. The Preoperative radiograph revealed periapical radiolucency in relation to mandibular right first premolar with loss of lamina dura, widening of the periodontal ligament space and supra bony pockets due to horizontal bone resorption (Figure 2).
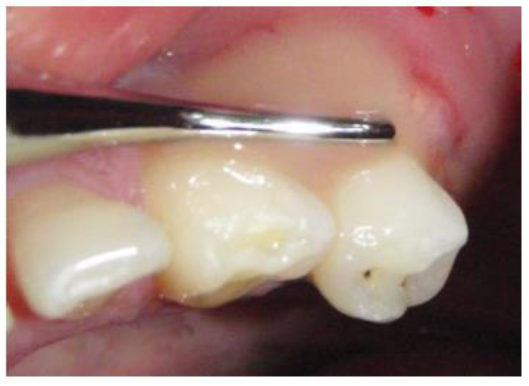
Figure 1: Preoperative photographe showing pus drainage
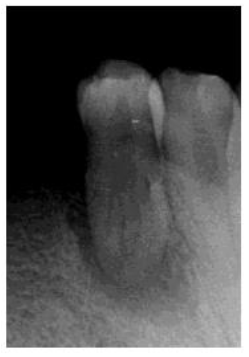
Figure 2: Preoperative radiograph
Based on the clinical and radiographic evidences, tooth #44 was diagnosed as necrotic pulp with exacerbation of acute periapical abscess, due to primary periodontal disease with secondary endodontic involvement (Class IV Simon classification). The first visit was an emergency visit where After local anesthesia (local nerve-block) with 2% lidocaine HCL with 1:100,000 epinephrine (Lidocaine; Cook-Waite Laboratories, Inc, New York, USA), the tooth no #44 was isolated with rubber dam and clamp (Hygenic, Coltene Whaledent, USA).The access cavity was initiated with a round high speed diamond bur (Dentsply Maillefer, Ballaigues, Switzerland), under the DOM (Opmi Pico, Carl Zeiss, Oberkochen, Germany), at a lower magnification. Initially two canal orifices were located and patency was ascertained using a size 10 K file. After careful inspection under higher magnification, third canal orifice was located. Access cavity refined using Endo Access bur number A0164 (Dentsply Maillefer, Switzerland) and ultrasonic tips (CPR, Dentsply Maillefer, Switzerland). Working length was established with the use of an Apex locator (Root ZX, J. Morita Inc).The three root canals (MB,DB and L) were cleaned and shaped using Profile NiTi system (Dentsply Maillefer, Ballaigues, Switzerland) in crown down technique to a size 35/04 for the buccal canals, and 40/04 for the lingual canal (apical preparation), The canal were sequentially irrigated using sodium hypochlorite (Chloraxid 5.25%, Cerkamed, Poland) and 17% EDTA during the cleaning and shaping procedure. The canals were dried with sterile paper points, and Calcium Hydroxide (Ultracal, Ultradent.com) was used as intracanal medicament for two weeks, then a thorough superficial scaling was done allowing drainage through the pocket using ultrasonic and hand scalers. Irrigation was done using normal saline. An regimen of Augmentin one gram (Glaxo Smith Kline) and Ibuprofen 400 mg (Ibuprofen Spimaco) was prescribed.
In the second appointment after two weeks, patient swelling subsided and the tooth was asymptomatic. The calcium hydroxide dressing was removed using (EDTA 17% solution) and NiTi hand K-files. The canals were irrigated again, dried with sterile paper points and the gutta-percha master cones (size 40, 4% taper for the lingual canal, size 35, 4% taper for buccal canals) were then cemented into the corresponding root canal with AH 26 sealer (Dentsply Maillefer, Ballaigues, Switzerland). The pulp chamber was temporary sealed with layer of Cavit (3M ESPE Dental AG, Seefeld/Oberbay,Germany), and light- cured glass ionomer filling material (Fuji II, GC, Japan). Final radiographs were taken to evaluate the quality of the performed endodontic treatment (Figure 3), and finally the tooth restored with amalgam restoration (Figure 4). The periodontal treatment was initiated three weeks after endodontic treatment, at this stage the acute inflammation was subsided and the gingiva appeared healthier and more easily handled. No significant changes were revealed in neither probing depth nor attachment level with no pus oozing. The tooth was Grade II mobility.
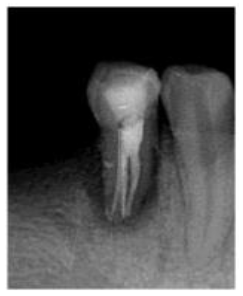
Figure 3: Post- obturation radiograph
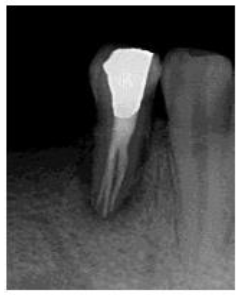
Figure 4: Post- restoration radiograph
After long block local anesthesia a modified Widman flap was initiated from the area of the lower right first molar till the distal surface of the lower right canine according to the technique presented by Ramfjord and Nissle 1974 [25]. In this technique an scalloped initial inverse incision was done 0.5 mm away from the gingival margin to the alveolar crest, the gingival flap was reflected followed by crevicular incision was made from the bottom of the pockets to the bone. A third incision was made in the interdental space using Orban knife to remove a triangular wedge of tissue containing the pocket lining. Tissue tags and granulation tissue was removed with area specific curettes. The root surfaces were scaled and planned. DuraDox 100 mg capsule (Doxycline Julphar industries) was mixed with Oraxine mouthwash (0.2% Chlrohexidine Gluconate Riyadh Pharma) and topically applied on the exposed root surfaces and left for 10 minutes. Irrigation with normal saline was done followed by suturing using interrupted direct sutures done at each interdental space using non-resorbable black braided silk 3-0 (Mersilk Soie Ethicon) suture wire. Periodontal packing using surgical pack (Coe-PAK.GC America inc.) was done. DuraDox 100 mg was started 2 days preoperative and continued for 8 days postoperative according to Slots et al [26]. Ibuprofin 400 mg was used as anti-inflammatory agent for 5 days with Oraxine mouthwash twice daily for 3 weeks. The patient was seen after one week pack and sutures removed followed by irrigation using hydrogen peroxide with superficial scaling to remove food debris. The patient was asked to do gentle brushing of the area using soft toothbrush. The patient was seen weekly in the first month postoperatively then every 3 months thereafter for 1 year (Figure 5). At the follow up visits probing depth, attachment level and degree of mobility were within normal limit.
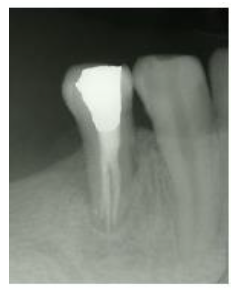
Figure 5: One year follow up
3. Discussion
Together with careful interpretation of radiographs, thorough knowledge of root canal anatomies essential for recognition and adequate treatment of teeth with different anatomical variations [1]. Several studies investigate the anatomy of mandibular first premolars, and show low incidences of three canals 0.5-2% [10,11]. This case report describes mandibular first premolars with three canals, two buccal and one lingual, with deep pockets, necrotic pulp and apical abscess. The effect of progressive periodontitis on the vitality of the pulp is controversial [20,27,28]. As long as the neuro-vascular supply of the pulp remains intact, prospects for survival are good. If lost due to periodontal disease, pulpal necrosis is about to occur [20]. This situation exists when the apical progression of periodontal disease is sufficient to open and expose the pulp to the oral environment by way of lateral canals or dentinal tubules. In these cases, bacteria originating from the periodontal pocket are the source of root canal infection. A strong correlation between the presence of microorganisms in root canals and their presence in periodontal pockets of advanced periodontitis has been demonstrated, indicating that similar pathogens may be involved in both diseases [29,30]. In this reported case the tooth was presented with deep pocketing and attachment loss more than 7mm in all the measured tooth surfaces with degree III mobility. The prognosis of such tooth was poor or even hopeless according to many authors and in such case depended mainly on the degree of contribution of the periodontal lesion in the destruction of the supporting tissue [12,31,32], a situation that cannot be evaluated on a clinical basis. Due to the patient desire to save the tooth and the functional need for the tooth in addition to the desire of the clinicians to keep this tooth with rare existence of three root canals in a lower first premolar, a decision was made to attempt maintenance of such tooth In the combined perio-endo lesions proper endodontic treatment as well as good periodontal management are key factors for successful healing of such lesions [33-35]. Rotestin and Simon as well as Chen et al. have reported a remission in periodontal bone loss after successful endodontic therapy alone in combined lesions, in addition endodontic treatment is highly predictable and its completion before periodontal treatment has a supportive effect on periodontal healing and aids in reliving any existing acute symptoms for the patient [22,36,37]. This lead clinicians to start endodontic treatment before periodontal therapy, accordingly the same is followed in this clinical case. Endodontic treatment initiated under surgical operating microscope has been found to be particularly helpful in locating and treating ‘extra’ canals [38]. The use of rotary Nickel-titanium rotary instruments has become an important adjunct in endodontic therapy. Despite the existence of one ever present risk factor- dental anatomy- shaping outcomes with these instruments are mostly predictable [39]. Even though Calcium hydroxide effectiveness in significantly increasing the number of culture-negative root canals after chemo mechanical preparation has been demonstrated to be inconsistent [40,41].Two visits endodontic treatment with the use of Calcium hydroxide as Intracanal medicament has been recommended to supplement the antibacterial effects of chemo mechanical procedures and maximize bacterial reduction [42-46]. In periodontal management modified Widman flap technique was applied as a treatment modality as it offers the possibility of establishing an intimate postoperative adaptation of the flap to the tooth surface in addition of providing proper access and visibility for proper mechanical debridement [25,47]. Moreover systemic antibiotic therapy combined with mechanical debridement appears valuable in the management of periodontal infection [48]. Doxycline was proved to concentrate in the periodontal tissues 2-10 times than serum with ability to inhibit growth of many of the periodontal pathogens and more readily absorption from the gastrointestinal tract [49,50]. In addition Doxycline was found to exert anti collagenase activity than the other members of the tetracycline group and may aid in bone regeneration [51,52]. Accordingly in this case report both topical and systemic Doxycline was used as an adjunctive to mechanical debridement. As all the pockets were of the suprabony type, no guided tissue regeneration nor bone augmentation was triad. No attempts were done to do bone sounding and probing during the first three months post-operative this not to disturb the still immature collagen fibers [53]. While complete regeneration of the lost supporting tissue due to root canal infection is expected after proper endodontic treatment, healing of the periodontal component is a more complicated problem and even with proper treatment the periodontal defect does not resolve to the same extent as the endodontic lesion [35]. During the healing stages of the periodontal pockets are invaded by a wide variety of cells from oral epithelium, gingival connective tissue, alveolar bone and periodontal ligament resulting in healing with either long junctional epithelium, repair, new attachment or less frequently regeneration of the lost supporting tissue [52,53]. In the present study and during the 6 & 12 months follow up periods the pocket measurements revealed remarkable improvement. Pocket depth is the distance from the gingival margin to the base of the pocket; it changes from time to time even in non-treated periodontal diseases because of changes in the gingival margin [55]. Thus such improvement in pocket depth measurements may be partially attributed to the recession usually following the periodontal treatment, the increased resilience of the gingiva after healing and actual regeneration and new attachment of part of the lost supporting periodontal tissue. On the other hand attachment level which represents the distance between cementoenamel junction and bone margin is a more better indicator in following healing after periodontal treatment [55]. Again a notable gain of attachment was detected during the follow up period indicating regeneration of some of the lost supporting periodontal tissues with some new attachment formation. This was supported in this study by cessation of tooth mobility with the remarkable improvement in the bone level with regain of the lamina dura and disappearance of the periodontal ligament widening. This dramatic improvement in the whole clinical parameters during the follow up periods indicated that most of the destruction in the supporting tissue was due to the pulpal infection and the periodontal disease acted only as an initiator for the lesion starting from one tooth surface only, most properly, the distal surface till the infection had reached the pulp tissues, from this point the pulpal infection got the leading role in supporting tissue destruction.
In conclusion it is essential to know that in perio- endo lesions, the endodontic treatment is the more predictable treatment, however, the success of such therapy is dependent on the completion of periodontal treatment and complete adequate management of both aspects is essential for successful long term results of such case.
References
- Macri E, Zmener O. Five canals in mandibular second premolar. J Endod 26 (2000): 304-305.
- Al-Fouzan KS. The microscopic diagnosis and treatment of a mandibular second premolar with four canals. Int Endod J 34 (2001): 406-410.
- Kamchan, Siu. Mandibular premolars with three root canals- two case reports. Int Endod 25 (1992): 261-264
- Slowey RR. Root canal anatomy. Road map to successful endodontics. Dent Clin North Am 23 (1979): 555-573.
- Ingle JI Endodontics. (3rd edtn), Lea & Febiger, Philadelphia, USA (1985).
- Mueller AH. Morphology of root canals. J Am Dent Assoc 23 (1936): 1698-1706.
- Weine FS. Endodontic Therapy. (5th edn), St. Louis: Mosby- Yearbook lnc (1996).
- Vertucci FJ. Root canal anatomy of the human permanent teeth. Oral surg oral med oral pathol 58 (1984): 589-599.
- Pineda F, Kuttler Y. Mesiodistal and buccolingual roentgenographic investigation of 7275 root canals. Oral surg oral med oral pathol 33 (1972): 101.
- Vertucci F. Root canal morphology of mandibular premolars. J Am Dent Assoc 97 (1978): 47-50.
- Walker RT. Root canal anatomy of mandibular first premolars in a southern Chinese population. Endod Dent Traumatol 4 (1988): 226-228.
- Wolf HF, Edith M, Rateitschak KH, et al. Atlas of Dental Medicine. Periodontology. (3rd edtn), Thieme. (2004): 445.
- Torabinjad M. Pulp and periradicular pathosis. In Walton RE, Torabinjad M, editors: Principles and practice of endodontics, Philadelphia, USA (2002).
- Simon J, Glick DH, Frank AL. The relationship of endodontic- periodontic lesions. J Clinic Periodontol 43 (1972): 202.
- Ehnevid H, Jansson, LE, Lindskog SF et al. Periodontal healing in relation to radiographic attachment and endodontic infection. Journal of Periodontology 64 (1993): 1199-1204.
- Miyashita H, Bergenholtz G, Wennstrom J. Impact of endodontic condition on marginal bone loss. Journal of Periodontology 69 (1998): 158-164.
- Ammors WF, Harrington GW. Periodontic- endodontic continum In: Carranza Clinical Periodontology. Newman MG, Takei HH, Carranza FA. (9th edtn), Saunders Elsevier Company, USA (2006).
- Harrnigton GW. The perio-endo question: differential diagnosis. Dent Clin North. Am 23 (1979): 673.
- Torabinjad M, Kiger RD. Histologic evaluation of dental pulp tissue of a patient with periodontal disease. Oral surg oral med oral pathol 59 (1985): 198.
- Langeland K, Rodrigues H, Dowden W. Periodontal disease, bacteria, and pulpal histopathology. Oral surg oral med oral pathol 37 (1974): 257-270.
- Nair PNR. Apical periodontitis a dynamic encounter between root canal infection and host repair. Periodontology 13 (2000): 121-148.
- Bergenholtz G, HasselgrenG, Endodontics and Periodontics. In clinical periodontology and implant Dentistry. Lindhe, Karring T, Lang N. (4th edtn), Blackwell Munksgaard (2003).
- Sunithav R, Emmadi P, Namasivayam A, et al. The periodontalendodontic continuum: A review. J Conserv Dent 11 (2008): 54-62.
- Nyman S, Lindhe J. Examination of patients with periodontal diseases. In clinical periodontology. Lindhe J, Karring T, Lang NP. Blackwell Munksgaard (2003).
- Ramfjorid SP, Nissle RP. The modified Widman flap. J perioontol 45 (1974): 601.
- Slots J, Mc Donald ES, Nowzari H. Infection aspects of periodontal regeneration. Periodontol 19 (2000): 164.
- Adriaens PA, De Boever JA, Loesche WJ. Bacterial invasion in root cementum and radicular dentin of periodontally diseased teeth in humans. A reservoir of periodontopathic bacteria. J Periodontol 59 (1988): 222-230.
- Adriaens PA, Edwards CA, De Boever JA, Loesche WJ. Ultrastructual observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodont 59 (1988): 493-503.
- Kipioti A, Nakou M, Legakis N, et al. Microbiological finding of infected root canals and adjacent periodontal pockets in teeth with advanced Oral surg oral med oral pathol 58 (1984): 213-220.
- Kobayashi T, Hayashi A, Yoshikawa R, et al. The microbial flora from root canals and periodontal pockets of nonvital teeth associated with advanced periodontitis. Int Endod J 23 (1990): 100-106.
- Novak KF, Goodman SF. Takei HH. In: carranza clinical periodontology. Newman MG, Takei HH, and Carranza FA. (9th edtn), Saunders Elsevier Company (2006).
- Solomon C, Chalafin H, Kellert M. The endodontic periodontal lesion, a rational approach to treatment. J Am Dent Assoc 126 (1995): 473-479.
- Schilder H. Endodontic-periodontal therapy. In: Grossman L, Endodontic Practice. (6th edtn), Lea and Febiger, Philadelphia, USA (1965).
- Simon P, Jacobs D. The so-called combined periodontal-pulpal problem. Dent Clin North Am 13 (1969): 45-52.
- Mandel E, Machton P, Torabinejad M. Clinical diagnosis and treatment of endodontic and periodontal lesions. Quintessence Int 24 (1993): 135-139.
- Rotstein I, Simon JH. Diagnosis, prognosis and decision making in the treatment of combined periodontal-endodontic lesions. Periodontol 34 (2004): 265-303.
- Chen SY, Wang HL, Glickman GN. The influence of endodontic treatment upon periodontal wound healing. J clin periodontol 24 (1997): 449-456.
- Carr GB. Microscopes in endodontics. J Calif Dent Assoc 20 (1992): 55-61.
- Peters OA. Current challenges and concepts in the preparation of root canal systems: A review. J Endodon 30 (2004): 559-597.
- Shuping GB, Ørstavik D, Sigurdsson A, et al. Reduction of intracanal bacteria using nickeltitanium rotary instrumentation and various medications. J Endod 26 (2000): 751-755.
- Waltimo T, Trope M, Haapasalo M, et al. Clinical efficacy of treatment procedures in endodontic infection control and one year follow-up of periapical healing. J Endod 31(2005): 863-866.
- Sjogren U, Figdor D, Spangberg L, et al. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J 24 (1991): 119-125.
- McGurkin-Smith R, Trope M, Caplan D, et al. Reduction of Intracanal bacteria using GT rotary instrumentation, 5.25% NaOCl, EDTA, and Ca(OH)2. J Endod 31 (2005): 359-363.
- Siqueira JF Jr, Guimaraes PT, Rocas IN. Effects of chemomechanical preparation with 2.5% sodium hypochlorite and intracanal medication withcalcium hydroxide on cultivable bacteria in infected root canals. J Endod 33 (2007): 800-805.
- Siqueira JF Jr, Magalhaes KM, Rocas IN. Bacterial reduction in infected root canals treated with 2.5% NaOCl as an irrigant and calcium hydroxide/camphorated paramonochlorophenol paste as an intracanal dressing. J Endod 33 (2007): 667-672.
- Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol 1 (1985): 170-175.
- Ramfjorid SP. Present status of the modified Widman flap procedure. J Periodontol 48 (1977): 558.
- Jolkovsky DL, Cianicio S. Chemotheraputic agents In: Carranza Clinical Periodontology. Newman MG, Takei HH, Carranza FA. (9th edtn), Saunders Elsevier Company, USA (2006).
- Baker PJ, Evans RT, Slots J et al. Susceptability of human oral anaerobic bacteria to antibiotic sutible for topical use. J Clin Periodontol 12 (1985): 201.
- Alger FA, Solt CW, Vuddhen Kanak S, et al. The histologic evaluation of new attachement in periodontally diseased human roots trated with tetracycline hydrochlorid and fibronectin. J Periodontol 61 (1990): 447.
- American Academy of Periodontology. Research, Sceince and Therapy Committe: Systemic antibiotic in periodontics. J Periodontol 75 (2004): 15-53.
- Goulb LN, Evan RT, Mc Namara TF et al. Anon antimicrobial tetracycline inhibits gingival matrix metalloproteainase and bone loss in prphrmonas gingivalis. Ann Sci 732 (1994): 96-106.
- Carranza FA, Takei H. Rational of periodontal treatment. In carranza clinical Periodontology. Newma MG, Takei HH, Carranza FA. (9th edtn), Saunders Elsevier Company, USA (2006).
- Melcher AH. On the repair potential of periodontal tissues. J Periodontol 47 (1976): 256.
- Carranza FA, Takih H. Clinical diagnosis In: Carranza Clinical Periodontology. Newman MG,Takei HH, Carranza FA. (9th edtn), Saunders Elsevier Company, USA (2006).
