Left Atrial Mechanics and Functional Capacity in HFpEF pts with Paroxysmal Atrial Fibrillation
Article Information
Ana Moya1, Monika Kodeboina1, Asim Katbeh1, Martin Penicka1, Sofie Verstreken1 and Marc Vanderheyden1*
1Cardiovascular Center, OLV Hospital, Aalst, Belgium
*Corresponding author: Marc Vanderheyden, Cardiovascular Center, OLV Hospital, Aalst, Belgium.
Received: 10 January 2023; Accepted: 21 January 2023; Published: 31 Janury 2023
Citation: Ana Moya, Monika Kodeboina, Asim Katbeh, Martin Penicka, Sofie Verstreken and Marc Vanderheyden. Left Atrial Mechanics and Functional Capacity in HFpEF pts with Paroxysmal Atrial Fibrillation. Behavior in Obese and Overweight Patients. Cardiology and Cardiovascular Medicine 7 (2023): 39-44.
View / Download Pdf Share at FacebookAbstract
Introduction: Patients (pts) with paroxysmal atrial fibrillation are at risk for developing heart failure with preserved ejection fraction(HFpEF) and often have an impaired exercise capacity. Since left atrial (LA) pressure plays a major role in the exercise intolerance, we aimed to characterize the contribution of resting LA mechanical properties, assessed by two-dimensional speckle tracking echocardiography upon exercise capacity.
Purpose: To evaluate relationship between LA mechanics, measured by LA strain (LAS) and parameters of exercise capacity in pts with paroxysmal atrial fibrillation.
Methods: The study included 54 pts (64 ± 10years, 76 % males) with dyspnea (NYHA≥II), paroxysmal atrial fibrillation and preserved LV ejection fraction (≥50%). The likelihood of HFpEF was estimated using H2FPEF score. During sinus rhythm, all patients underwent speckle tracking echocardiography and cardiopulmonary exercise testing (CPET). Peak oxygen uptake (VO2peak) served as measure of functional capacity and ventilation/carbon dioxide output slope(VE/VCO2) as surrogate of ventilation/perfusion mismatch.
Results: LA contractile strain(S) and strain rate(SR) showed significant correlation with VO2peak(both p < 0.05). LA reservoir, conduit and contractile LAS, all had significant relationship with VE/VCO2 slope (all p<0.050). Pts with LASR above the median had significantly higher VE/VCO2 (p=0.025) and lower VO2peak(p=0.010). In contrast, no correlations were observed between exercise parameters and LA volumes or any other echocardiographic indices.
Conclusions: In HFpEF VO2peak and VE/VCO2 are closely related to LA contractile strain, suggesting that abnormalities in LA mechanics may contribute to t
Keywords
Atrial Fibrillation; Functional Capacity; HFpEF; Left Atrial Strain
Article Details
List of Abbreviations:
LAS- Left Atrial Contractile Strain; LASR- Left Atrial Contractile Strain Rate; LASr- Left Atrial Reservoir Strain; LAScd- Left Atrial Conduit Strain; LASct- Left Atrial Contractile Strain; GLS- Global Longitudinal Strain
1. Introduction
Approximately half of the patients with heart failure have HFpEF [1] and it’s inceidence is increasing steadily over the last years making it a global health problem [2]. Interestingly a subgroup of pts with paroxysmal atrial fibrillation is at risk for developing HFpEF. Whereas the pathophysiology of HFpEF has been attributed to increased left ventricular (LV) and vascular stiffness, recent findings point also at the contributing role of left atrial (LA) mechanical dysfunction to the clinical presentation [3]. Indeed, the left atrium modulates LV filling and alterations in atrial functional parameters have been detected at the earliest stage of HFpEF [4,5]. Apart from LA endocrine failure with deficient ANP production and ANP resistance, LA remodelling and LA regulatory failure with orthosympathic overload and excessive vasopressine, mechanical failure of the left atrium itself contributes directly to the complex pathophysiological mechanism of HFpEF [3,6]. In addition to 2D-echocardiography being a routine tool to evaluate LA function, speckle tracking echocardiography appears to be more sensitive and specific method for LA functional assessment [7,8]. It enables to dissect LA function in three phases of reservoir, conduit and contractile function, and provides deeper insight in the different components of LA mechanics. In HFpEF pts, studies have demonstrated that compared to healthy controls, LA reservoir as well as conduit and pump function are impaired [3,9]. HFpEF pts are characterized by exertional dyspnoea and impaired exercise capacity which can be quantified by cardiopulmonary exercise testing (CPET) [10]. It offers the most objective and comprehensive assessment of functional capacity and provides important information about the individual functional capacity [10]. As we hypothesise that LA mechanics may interfere with functional capacity in HFpEF pts, the present study was set up to assess the interrelationship between 2D and strain echocardiographic parameters of LA function and exercise capacity, determined by CPET in a group of pts with paroxysmal atrial fibrillation with dyspnoea and a H2FPEF score ≥5 suggestive for HFpEF.
2. Methods
2.1 Study Population
The study population consisted of 54 consecutive patients with limiting exertional dyspnoea (NYHA≥II) and paroxysmal atrial fibrillation undergoing maximal cardiopulmonary exercise test prior to pulmonary vein isolation. Patients with acute coronary syndrome, moderate valvular heart disease, hypertrophic or restrictive cardiomyopathy or reduced LV ejection fraction (<50%), were excluded. Likelihood of HFpEF was assessed using H2FPEF score, which allows discriminating HFpEF from non-cardiac causes of dyspnea [11]. All pts had a H2FPEF score ≥5 suggesting high probability of HFpEF [11]. Nt-proBNP (Roche Diagnostics) levels were measured in venous blood at rest. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Onze Lieve Vrouw Hospital, Aalst, Be, OLV study number: varia 10/D).
2.2 Exercise Testing
All patients underwent maximal cardiopulmonary exercise test before enrolment in the cardiac rehabilitation program and were free of exercise-limiting co-morbidities, such as cerebrovascular disease, musculoskeletal impairment or vascular disease of the lower extremities. Patients could only be included if they performed a maximal exercise test with a respiratory exchange ratio (RER) >1. The protocol used for the exercise testing has been reported previously. Subjects were exercised on a computer-driven cyclo-ergometer (Marquette Case 8000, Marquette Electronics, Milwaukee, Wisc., USA) using a ramp protocol starting at 20 watts with gradual increase of 10 watts every minute. The 12 lead ECG and heart rate were recorded continuously during the test. Cuff blood pressure was measured every two minutes of the exercise test with a manual manometer. Subjects were exercised to their self-determined maximal capacity or until the physician stopped the test because of significant symptoms, such as chest pain or dizziness, potentially dangerous arrhythmias, ST-segment deviations, marked systolic hypotension or hypertension.
2.3 Respiratory Gas Measurements
Continuous respiratory gas measurements were obtained by using a Medical Graphics Cardiopulmonary Exercise System (Medical Graphics, Minneapolis, Minn., USA). The oxygen consumption (VO2), carbon dioxide production (VCO2), minute ventilation (VE), tidal volume, respiratory rate and mixed expiratory carbon dioxide concentration were continuously measured on a breath-by-breath basis. In addition, several derived variables such as the respiratory exchange ratio (RER) and the ventilatory equivalent for oxygen (VE/VO2) and CO2 (VE/VCO2) were calculated. Peak VO2 was expressed as the highest attained VO2 during the final 30 seconds of exercise. VE/VCO2-ratio was determined by linear regression analysis of the relation between VE and VCO2 during exercise, with data obtained over the complete duration of the exercise test (including respiratory compensation). Flow meters and gas analysers were calibrated for accuracy and linearity with a syringe of known volume and with precisely analysed gas mixtures daily.
2.4 Echocardiography
A comprehensive 2D transthoracic echocardiographic examination was performed using Vivid E95 (GE HealthCare, Horten, Norway) ultrasound system. All acquired images were stored digitally for offline analysis using a commercially available software (EchoPac, GE HealthCare). All examinations were recorded during sinus rhythm and analyzed by the same operator. Average of at least 3 beats was taken for each measurement. Blood pressure and heart rate were recorded during each examination. The biplane Simpson method was used to assess LV volumes and ejection fraction [12]. Maximum LA volume and LA emptying fraction were calculated from the apical 4- and 2-chamber views using the area-length method [12]. LV and RV global longitudinal strain (GLS) were assessed using speckle tracking technique in views optimized for each chamber and at frame rate of >60 Hertz [12, 13]. Assessment of longitudinal LAS and strain rate (SR) were performed as recommended [14]. In brief, optimized apical 4- and 2-chamber views were recorded during breath hold. LA endocardial borders were traced manually in all views. Region of interest was manually adjusted and tracking quality was previewed before generating LAS and LASR curves. The LA reservoir (LASr), conduit (LAScd) and contractile (LASct) strain and SR were assessed as average of segmental values in apical views using the onset of QRS as a reference point [14,15].
2.5 Statistics
All results are expressed as mean ± SD. Student T-test and a Spearman correlation coefficient were used for appropriate comparisons. Statistical significance was set at a two-tailed probability level of less than 0.05.
3. Results
3.1 Baseline Demographics and Echocardiography
Clinical and echocardiographic characteristics of the study population are summarized in table 1. Demographic characteristics demonstrate a high incidence of hypertension whereas diabetes pts (4%) and patients with morbid obesity (BMI: 28,1 ± 5,5 kg/m2) were relatively rare. By definition H2FPEF score was elevated, ranging between 5 and 9 points (mean 7) suggestive for HFpEF. Also, the Nt-proBNP levels were elevated (341 ± 383 pg/ml). All patients showed a non-dilated left ventricle with preserved contractile function as evidenced by LV-EF and GLS. Pulmonary pressures were within normal limits (Table 1). Table 2 summarizes the CPET characteristics. All individuals performed a maximal exercise test as evidenced by a RER >1.10 with a peak VO2 of 21,6 6,4 ml/kg/min and a VE/VCO2 slope of 30,1 4,9.
|
All Patients (n=54) |
|
|
CLINICAL CHARACTERISTICS |
|
|
Age (years) |
64 ± 10 |
|
BMI (kg/m2) |
28,1 ± 5,5 |
|
Male sex (%) |
76 |
|
Hypertension(> 2 antihypertensive medications) (%) |
43 |
|
Smoker (%) |
3 |
|
Diabetes (%) |
4 |
|
IHD (%) |
5 |
|
COPD (%) |
10 |
|
ECHO PARAMETERS |
|
|
LVEDVI (mL/m2) |
54,2 ± 10,9 |
|
LVEDV (mL) |
110,2 ± 23,1 |
|
LVESVI (mL/m2) |
22,1 ± 6,8 |
|
LVESV (mL) |
44,6 ± 13,6 |
|
LVEDD (mm) |
49,7 ± 5,0 |
|
LVMI (g/m2) |
91,9 ± 28,4 |
|
LVEF (%) |
62,2 ± 8,0 |
|
LV GLS (%) |
- 20,0 ± 2,8 |
|
RV GLS (%) |
- 21,9 ± 5,3 |
|
E/A |
1,35 ± 0,44 |
|
Mean E/e´ |
8,55 ± 2,38 |
|
PASP (mm Hg) |
32,1 ± 7,0 |
|
RV TAPSE (mm) |
24,9 ± 4,3 |
|
BIOMARKERS |
|
|
Nt-proBNP (pg/ml) |
341 ± 383 |
Table 1: Study Population: Baseline demographics and echocardiography characteristics.
Data- are expressed as mean ± standard deviation or %; LV - Left Ventricular; LVEDV- LV End-Diastolic Volume; LVESV- LV End-Systolic Volume; LV GLS - LV Global Longitudinal Strain; IHD- Ischemic Heart Disease; COPD- Chronic Obstructive Pulmonary Disease; LVESVI- indexed LVESV; LVEDVI- indexed LVEDV; LVEF- LV Ejection Fraction; LVEDD- LV End-Diastolic Diameter; LVMI- LV Mass Index; sPAP- Systolic Pulmonary Artery Pressure; RV GLS - Right Ventricular Global Longitudinal Strain; BMI - Body Mass Index.
|
All Patients (n=54) |
|
|
VO2 (mL/min) |
1867 ± 658 |
|
VO2/Kg (mL/min/kg) |
21,6 ± 6,4 |
|
Peak RER |
1,1 ± 0,1 |
|
Peak Heart rate (bpm) |
126 ± 21 |
|
HRR |
14,3 ± 3,6 |
|
VE/VCO2 slope |
30,1 ± 4,9 |
Table 2: Study Population: Cardiopulmonary Characteristics.
Data are expressed as mean ± standard deviation. Peak RER indicates peak respiratory exchange ratio, HRR heart rate reserve.
3.2 Left Atrial Mechanical Function and Exercise Capacity
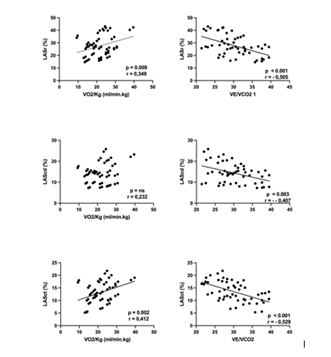
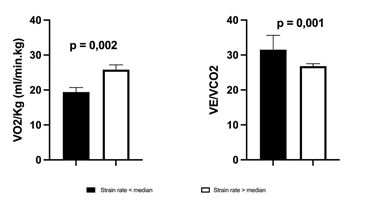
Indices of LA size and function are depicted in table 3. Left atrial volume index was increased while LA emptying fraction was within normal limits. In contrast, LAS showed a significant reduction in all components of LA phasic function. Out of all the echocardiographic parameters, contractile and reservoir LAS (Figure 1) showed significant correlation with VO2peak whereas the VE/VCO2 slope was inversely related to reservoir, conduit and contractile LAS (Figure 1). A significant correlation was observed between LASR and VO2peak (r=0,562, p=0.012) and VE/VCO2 slope (r=-0,598, p=0.009). Pts with LASR below the median were characterized by a significantly higher VE/VCO2 slope(31,5 ± 4,1 vs 25,68 ± 3,46; p=0.001) and lower peak VO2(19,4 ± 1,3 vs 25,8 ± 1,4 ml.min-1.kg-1.; p=0.002) (Figure 2). Finally, no correlations were observed between exercise parameters and LA volumes, LA emptying fraction or Doppler parameters of diastolic function.
|
All Patients (n=54) |
|
|
LA volume (mL) |
73,6 ± 22,2 |
|
LAVI (mL/m2) |
36,2 ± 6,5 |
|
LA ejection fraction (%) |
54,8 ± 9,2 |
|
LA Reservoir Phase |
|
|
Global strain-LASr (%) |
27,2 ± 8,1 |
|
Global strain rate (/s) |
-1,3 ± 0,3 |
|
LA Conduit Phase |
|
|
Global strain-LAScd (%) |
14,0 ± 4,7 |
|
Global strain rate (/s) |
-1,1 ± 0,4 |
|
LA Contractile Phase |
|
|
Global strain-LASct (%) |
13,2 ± 4,3 |
|
Global strain rate (/s) |
-1,5 ± 0,5 |
Table 3: Study Population: Echocardiographic characteristics of left atrium.
Data are expressed as mean ± standard deviation or %. LA = left atrial, LAVI- LA Volume Index; LAS- Left Atrial Strain; LASR- Left Atrial Strain Rate.
4. Discussion
The findings of the present study can be summarized as follows: [1] Left atrial mechanical function is impaired in HFpEF pts with paroxysmal atrial fibrillation [2]. The close relationship between exercise parameters and indices of left atrial strain suggest that in HFpEF pts with paroxysmal atrial fibrillation left atrial mechanical dysfunction plays a key role in the pathophysiology, thereby contributing to the blunted exercise capacity observed in these pts [3]. Based upon these observations we speculate that left atrial strain markers can be used as an echocardiographic surrogate for the assessment of functional capacity in HFpEF pts with paroxysmal atrial fibrillation. The left atrium plays a key role in maintaining optimal cardiac performance. Through its reservoir, conduit and pump function it modulates LV filling, maintains LV preload and cardiac output [16,17]. In addition, the compliance characteristics of the left atrium assures that the lungs remain free of congestion [3]. Recent studies have demonstrated that in HFpEF more parameters related to reduced left atrial function rather than those reflecting left atrial remodeling have diagnostic and prognostic value [18]. Left atrial mechanics correlate with left heart filling pressures, PA pressures, PA elastance and cardiac index at rest as well as during exercise [4,9] and a reduced atrial functional reserve during handgrip exercise is able to predict a blunted exercise capacity in HFpEF [19]. Our study is in line with these observations. The reported correlation between LA reservoir strain and peak oxygen uptake and ventilatory efficiency suggest that abnormalities in left atrium mechanics might impede the augmentation of cardiac output during exercise and contribute to the exercise intolerance, observed in this study population. In addition, it shows that abnormalities in LA function, measured at rest can decipher and identify the dynamic responses to exercise. The interplay between LA dilatation, LA mechanical dysfunction and atrial fibrillation is critical. Atrial fibrillation makes the atrium susceptible to LA dilatation which in itself is an adaptive change in HFpEF due to heightened LV filling pressures. On the other hand, LA dysfunction as well as heart failure and death have all been associated in previously asymptomatic elderly HFpEF subjects with enlarged LA volume [3]. We were unable to decipher any correlation between LA volume or emptying fraction and exercise capacity which similarly to previous observations indicates that LA mechanical function more than LA structure is the predominant correlate of abnormal hemodynamics in HFpEF [16]. Moreover, the highest correlation was observed between LA contractile strain and exercise parameters which emphasizes that the atrial booster function importantly contributes to exercise capacity [9].
5. Limitations
Our results should be interpreted considering some limitations. First, this was a single-center study with a relatively small number of patients, thus our findings will need to be confirmed in further investigations with a larger sample size. Second, the assessment of LA strain is vendor dependent, a pitfall which has partly been overcome by using vendor independent software. Nevertheless, the cut-off value for abnormal LA strain is often not well defined. This implies that the absolute values for LA strain should be interpreted with caution [5,7,20]. Finally, since measurements were obtained at a single time point, before pulmonary vein isolation and a control group was not included, further longitudinal studies are required to investigate the impact of pulmonary vein isolation upon LA mechanics and its relation to exercise capacity.
6. Clinical Implications and Conclusion
In our study, we demonstrated that impaired LA mechanics as measured by LA strain might play a role in the pathophysiology of HFpEF and associated with decreased peak oxygen consumption and ventilatory efficiency. Further prospective studies to validate indices of LA mechanics in predicting exercise intolerance in HFpEF are warranted.
Statements and Declarations
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
All authors have contributed substantially to the work reported and have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the In-stitutional Review Board (or Ethics Committee) of Onze Lieve Vrouw Hospital, Aalst, Be).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
References
- Owan T, Hodge D, Herges R, et al. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction, New Engl J Med 355 (2006): 251-259.
- Zakeri R, Chamberlain A, Roger V, et al. Temporal Relationship and Prognostic Significance of Atrial Fibrillation in Heart Failure Patients With Preserved Ejection Fraction, Circulation 128 (2013): 1085-1093.
- Khan M, Memon M, Murad M, et al. Left atrial function in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail 22 (2020): 472-485.
- Obokata M, Negishi K, Kurosawa K, et al. Incremental Diagnostic Value of LA Strain With Leg Lifts in Heart Failure With Preserved Ejection Fraction, Jacc Cardiovasc Imaging 6 (2013): 749-758.
- Singh A, Addetia K, Maffessanti F, et al. LA Strain Categorization of LV Diastolic Dysfunction., Jacc Cardiovasc Imaging 10 (2016): 735-743.
- Triposkiadis F, Pieske B, Butler J, et al. Global left atrial failure in heart failure, Eur J Heart Fail 18 (2016): 1307-1320.
- Morris D, Dungen H, Boldt L. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study, European Hear J - Cardiovasc Imaging 16 (2014): 364-372.
- Sugimoto T, Robinet S, Dulgheru R, et al. Study, Echocardiographic reference ranges for normal left atrial function parameters: results from the EACVI NORRE study., European Hear J Cardiovasc Imaging 19 (2018): 630-638.
- Telles F, Nanayakkara S, Evans S, et al. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction, Eur J Heart Fail 21 (2019): 495-505.
- Malhotra R, Bakken K, D’Elia E, et al. Cardiopulmonary Exercise Testing in Heart Failure, Jacc Hear Fail 4 (2016): 607-616.
- Reddy Y, Carter R, Obokata M, et al. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction., Circulation (2018).
- Lang R, Badano L, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging, European Hear J - Cardiovasc Imaging 16 (2015): 233-271.
- Lang R, Badano L, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging, J Am Soc Echocardiog 28 (2015): 1-39.e14.
- Badano L, Kolias T, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging., European Hear J Cardiovasc Imaging 19 (2018): 591-600.
- Mor-Avi V, Lang R, Badano L, et al. Current and Evolving Echocardiographic Techniques for the Quantitative Evaluation of Cardiac Mechanics: ASE/EAE Consensus Statement on Methodology and Indications, J Am Soc Echocardiog 24 (2011): 277-313.
- Obokata M, Borlaug BA. Left atrial dysfunction: the next key target in heart failure with preserved ejection fraction, Eur J Heart Fail 21 (2019): 506-508.
- Obokata M, Reddy YNV, Borlaug BA. Diastolic Dysfunction and Heart Failure With Preserved Ejection Fraction: Understanding Mechanisms by Using Noninvasive Methods., Jacc Cardiovasc Imaging. 13 (2019): 245-257.
- Freed BH, Daruwalla V, Cheng JY, et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction, Circulation Cardiovasc Imaging 9 (2016): e003754.
- Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular Features of Heart Failure With Preserved Ejection Fraction Versus Nonfailing Hypertensive Left Ventricular Hypertrophy in the Urban Baltimore Community The Role of Atrial Remodeling/Dysfunction, J Am Coll Cardiol 49 (2007): 198-207.
- Obokata M, Borlaug BA. Left Ventricular Filling Pressures in Heart Failure With Preserved Ejection Fraction: Is the Tail Now Wagging the Dog?, JACC. Heart Failure 5 (2017): 802-804.