Lactobacillus plantarum SN13T Cells Improve Hepatic Dysfunction And Fecal Microbiota: A Randomized Pilot Study
Article Information
Fumiko Higashikawa1, Narandalai Danshiitsoodol1, Keishi Kanno2, Ryoko Ishida3, Susumu Tazuma2, 4, and Masanori Sugiyama1*
1Department of Probiotic Science for Preventive Medicine, Graduate School of Biomedical and Health Sciences, Hiroshima University, Kasumi 1-2-3, Minami-Ku, Hiroshima 734-8551, Japan
2Department of General Internal Medicine, Hiroshima University Hospital, Kasumi 1-2-3, Minami-Ku, Hiroshima 734-8551, Japan
3Department of Community Based Medical System, Graduate School of Biomedical and Health Sciences, Hiroshima University, Kasumi 1-2-3, Minami-Ku, Hiroshima 734-8551, Japan
4JA Onomichi General Hospital, Hirahara 1-10-23, Onomichi 722-8508, Japan
*Corresponding author: Professor Masanori Sugiyama, Department of Probiotic Science for Preventive Medicine, Graduate School of Biomedical and Health Sciences, Hiroshima University, Kasumi 1-2-3, Minami-Ku, Hiroshima 734-8551, Japan;
Received: 23 September 2020; Accepted: 03 October 2020; Published: 05 November 2020
Citation: Fumiko Higashikawa, Narandalai Danshiitsoodol, Keishi Kanno, Ryoko Ishida, Susumu Tazuma, and Masanori Sugiyama. Lactobacillus plantarum SN13T Cells Improve Hepatic Dysfunction And Fecal Microbiota: A Randomized Pilot Study. Archives of Clinical and Biomedical Research 4 (2020): 605-625.
View / Download Pdf Share at FacebookAbstract
The liver dysfunction is a worldwide health problem. It has been suggested that an imbalance of gut microbiome is associated with a variety of diseases. The goal of the present clinical trial is to evaluate the effectiveness of Lactobacillus plantarum SN13T on hepatic function and fecal microbiota. This study enrolled 22 subjects, aged between 20 and 70 years, and who had any of the following conditions: 40<AST<100 U/L, 40<ALT<100 U/L, or 70<γ-GTP<210 U/L for males; 30<γ-GTP<90 U/L for females. The subjects were assigned to the live or heat-killed SN13T group. The intake period was 16 weeks followed by an 8-week follow-up period. Although no difference was observed between the two groups in the changes of AST, ALT, and γ-GTP, the subgroup analyses of subjects with over a certain level at baseline showed significant decreases in AST (-14.6 U/L, P=0.028) and ALT (-15.4 U/L, P=0.023) by SN13T, regardless of live or dead. The fecal microflora analysis showed an increase of Firmicutes and the decreases of Bacteroidetes and Fusobacteria in both groups. Bifidobacterium was increased only in the live SN13T group. In conclusion, Lactobacillus plantarum SN13T alters the composition of gut microbiota and improves liver function in subjects with mild liver dysfunction.
This clinical trial was registered with University hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) as UMIN000027440.
Keywords
A plant-derived lactic acid bacterium; Mild liver dysfunction; AST; ALT
A plant-derived lactic acid bacterium articles; Mild liver dysfunction articles; AST articles; ALT articles
Article Details
Abbreviations:
LAB: lactic acid bacteria; Lb: Lactobacillus; SN13T: lactobacillus plantarum SN13T; CTCAE v4.0: Criteria for Adverse Events version 4.0
Introduction
Alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) are major health concerns in industrialized countries. The increase of NAFLD is in parallel with the increasing prevalence of obesity and other components of the metabolic syndrome [1]. NAFLD is the most common liver disease in the United States, and NAFLD prevalence was 30% according to the National Health and Nutrition Examination Survey (NHANES) 1999-2012 [2]. In Asia, the NAFLD prevalence was approximately 34% between 2012 and 2017 [3]. A fatty liver is generally caused by the excess intake of alcohol and food. Therefore, the treatments for fatty liver are mainly lifestyle changes, such as weight loss, exercise, and restriction of alcohol intake, as well as drug therapy for co-morbidity, including insulin resistance and hyperlipidemia [4]. However, no standardised pathophysiologically directed therapy is currently available [4].
Recent studies suggest that intestinal dysbiosis—an imbalance of the microbiome—is associated with various diseases, including obesity, diabetes mellitus, inflammatory bowel disease, celiac disease, colorectal cancer, Alzheimer’s disease, and multiple sclerosis [5-7]. Thus, the improvement of gut microbiota is one of the key targets to overcome these diseases.
Lactic acid bacteria (LAB) are known as probiotics and health-beneficial bacteria and are traditionally used to produce fermented foods, such as yogurt, cheese, pickles, and kimchi. Some LAB strains have beneficial effects on constipation, immunity, cancer, obesity, and ulcerative colitis; they also contribute to reducing serum lipid [8-12], and are likely to improve gut microflora composition [13,14].
We have previously shown that a plant-derived lactic acid, Lactobacillus plantarum SN13T, improves hepatic function, according to the subgroup analyses of subjects with elevated AST, ALT, or γ-GTP [8]. However, since the previous study was designed for persons with a gastrointestinal complaint, in this study, we conducted another clinical trial at a small scale, enrolling subjects with slightly elevated liver function test values to determine whether SN13T strain consumption results in the improvement of liver function.
Materials and Methods
Materials and participants
A plant-derived Lactobacillus plantarum SN13T has been isolated from the banana leaf. Both experimental beverages produced as carrot juices were kindly provided by Nomura Dairy Products Co., Ltd. Each 120 mL beverage was individually packed without a package label.
Subjects suitable for the study criteria were recruited from Hiroshima city and its provincial area Japan. The inclusion criteria were as follows: (1) male or female between the ages of 20 and 70 years and (2) fit into any of the following ranges: 40<AST<100 U/L, 40<ALT<100 U/L, or 70<γ-GTP<210 U/L for males; 30<γ-GTP<90 U/L for females. The exclusion criteria were as follows: (1) diagnosis of virus hepatitis, autoimmune hepatitis, or cirrhosis of the liver; (2) taking medicines for chronic disease or continuously taking antiflatulent, antibiotic, or purgative drugs that may affect the intestinal flora; (3) allergy to milk; (4) participation in any clinical trial within 90 days of the commencement of the trial; (5) pregnant or nursing a child; or (6) judged as ineligible by clinical investigators. Tests for virus hepatitis were performed by measuring the HCV antibody and HBs antigen, and the possibility of autoimmune hepatitis was excluded using an antinuclear antibody test. The Ethics Committee of Hiroshima University approved the clinical study. The study was performed in accordance with the guidelines of the Helsinki Declaration, and all participants provided written informed consent prior to enrollment.
Study design
The current clinical trial was a randomized, double-blind, and parallel study conducted at Hiroshima University Hospital from July 2017 to Mar 2018. The eligible subjects were enrolled by an investigator and randomly assigned to one of two groups, the live or heat-killed SN13T group. The allocation ratio was 1:1, and the allocation sequence was generated by a non-clinical staff using a Microsoft Excel randomization function. The assignment was performed by the same staff. The subjects, clinical staff, and data analyst were blinded. Primary outcomes were 16-week changes in ALT, AST, and γ-GTP. The secondary outcomes were 16-week changes in total cholesterol, HDL cholesterol, triglyceride, fasting blood glucose, insulin, HOMA-R (homeostasis model assessment ratio), TNF-α (tumor necrosis factor-α), intestinal flora, and defecation frequency.
The subjects each drank 120 mL of the experimental beverage containing 1.2×1011 of either the live or heat-killed SN13T daily, at any time of day, for 16 weeks. The subjects were instructed to (1) keep their ordinary lifestyle and not to eat or drink too much during the study period; (2) keep a dairy of their beverage consumption, healthy condition, medicines, supplements, and defecation frequency; (3) avoid eating too much fermented food, such as yogurt, kimchi, or natto; (4) record the contents of their meals, including snacks and alcoholic drinks, for 3 days before the examination; and (5) refrain from donating blood. Clinical visits were scheduled for weeks 0, 4, 8, 16, and 24. A biochemical blood test, hematological assessment, blood pressure check, and physiological test were carried out at every visit. The blood samples were taken after over 9 h of fasting. Feces was collected at week 0 and week 16 within 3 days of the clinical visit for gut microbiome analyses. In the case that the subjects took antibiotics, feces collection was postponed at least 7 days from the last antibiotic administration. The serum TNF-αlevel was measured at week 0 and 16. The extraction of DNA from fecal samples was done by the method using lytic enzymes [15, 16]. Metagenome analyses of the DNA extracted from the intestinal bacteria were outsourced to a University start-up company, MyMetagenome Co., Ltd (Tokyo, Japan). Metagenome analyses were performed as previously described [15, 16]. Briefly, the V1-V2 region of bacterial 16S rRNA-encoding gene was amplified by 27Fmod 5’-AGRGTTTGATYM TGGCTCAG-3’ and 338R 5’-TGCTGCCTCCCGTAGG AGT-3’ primers. The amplified DNA fragment was sequenced using the MiSeq benchtop sequencer according to the Illumina protocol. All 3,000 filter-passed reads were rearranged in descending order according to the quality value and then clustered into OTUs with a 97% pairwise-identity cutoff using the UCLUST program (https://www.drive5.com, version 5.2.32).
For safety outcomes, newly emerged or a worsened case in the grade of the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0) was counted as an adverse event.
Statistical analysis
We determined the sample size to be 22 for a small pilot study to compare the effects of live and dead cells of the SN13T grown in carrot juice on liver function. Since 16-week changes in AST, triglycerides, and HOMA-R did not follow a normal distribution based on the Shapiro–Wilk test, these parameters were analyzed after logarithmic transformation. The changes during intake of the SN13T-fermented beverage were compared between or within the groups using Student’s t-test or paired t-test. The subgroup analyses were exploratory performed to evaluate the effects of baseline values on the reduction of AST or ALT (>=28 U/L at baseline) and AST/ALT ratio (>1.0 or <=1.0 at baseline). The data analyses were carried out as a full-analysis set (FAS), and the missing data were filled in using the multiple-imputation method, creating 20 datasets. Fisher’s exact test was applied for categorical variables to determine the difference in adverse events between groups. The statistical analyses were performed using the IBM SPSS (Statistical Package for the Social Sciences) Statistics 22. The data are expressed as the mean ± SD in tables and mean ± SE in figures, and P<0.05 was considered significant.
Results
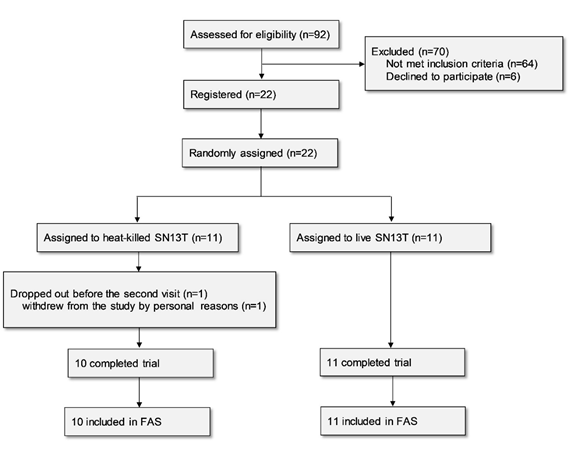
Twenty-two subjects were enrolled in the study for assessment of the efficacy of the beverage containing live or heat-killed Lb. plantarum SN13T on liver function. One subject belonging to the live SN13T group dropped out before the second visit for personal reasons and thus was excluded from the analysis based on the predefined FAS rule, since no data existed after beverage intake (Fig. 1).
The background data are shown in Table 1. Only the LDL/HDL ratio was significantly different between the groups at baseline (P=0.039).
|
All (n=21) |
Heat-killed SN13T |
Live SN13T |
||||
|
Age (y) |
54.2 ± 9.2 |
55.2 ± 10.9 |
53.4 ± 7.8 |
|||
|
Sex (n [%]) |
||||||
|
Male |
8 (38.1) |
3 (30.0) |
5 (45.5) |
|||
|
Female |
13 (61.9) |
7 (70.0) |
6 (54.5) |
|||
|
Height (cm) |
161 ± 9 |
160 ± 10 |
161 ± 8 |
|||
|
Body weight (kg) |
60.5 ± 10.4 |
59.1 ± 9.9 |
62.1 ± 11.2 |
|||
|
BMI (kg/m2) |
23.4 ± 3.5 |
22.7 ± 3.1 |
24.1 ± 3.9 |
|||
|
Body fat percentage (%) |
28.1 ± 8.3 |
27.3 ± 8.5 |
28.3 ± 8.4 |
|||
|
Systolic blood pressure (mmHg) |
116 ± 13 |
117 ± 14 |
116 ± 14 |
|||
|
Diastolic blood pressure (mmHg) |
72.1 ± 9.2 |
71.5 ± 9.7 |
72.8 ± 9.1 |
|||
|
Heart rate (beats per min) |
69.4 ± 8.2 |
69.2 ± 9.5 |
69.7 ± 7.0 |
|||
|
White blood cell count (×103/mL) |
5.38 ± 1.65 |
4.83 ± 1.58 |
5.88 ± 1.61 |
|||
|
Red blood cell count (×106/mL) |
4.54 ± 0.47 |
4.31 ± 0.44 |
4.75 ± 0.41 |
|||
|
Haemoglobin (g/dL) |
14.2 ± 1.4 |
13.5 ± 1.1 |
14.8 ± 1.3 |
|||
|
Haematocrit (%) |
43.9 ± 3.4 |
42.5 ± 2.9 |
45.2 ± 3.4 |
|||
|
Platelet count (×104/mL) |
24.5 ± 5.1 |
24.3 ± 4.4 |
24.7 ± 5.8 |
|||
|
AST (U/L) |
32.3 ± 19.1 |
34.3 ± 23.9 |
30.2 ± 12.9 |
|||
|
ALT (U/L) |
32.5 ± 24.1 |
25.0 ± 10.5 |
40.7 ± 32.0 |
|||
|
γ-GTP (U/L) |
72.8 ± 38.8 |
74.5 ± 39.7 |
70.9 ± 39.8 |
|||
|
Alkaline phosphatase (U/L) |
219 ± 62 |
204 ± 60 |
232 ± 64 |
|||
|
Total protein (g/dL) |
7.47 ± 0.48 |
7.37 ± 0.55 |
7.56 ± 0.42 |
|||
|
Total bilirubin (mg/dL) |
0.72 ± 0.21 |
0.68 ± 0.15 |
0.76 ± 0.25 |
|||
|
Albumin (g/dL) |
4.56 ± 0.25 |
4.42 ± 0.23 |
4.69 ± 0.20 |
|||
|
Uric acid (mg/dL) |
5.81 ± 1.64 |
5.08 ± 0.93 |
6.47 ± 1.90 |
|||
|
Creatinine (mg/dL) |
0.686 ± 0.169 |
0.650 ± 0.213 |
0.718 ± 0.117 |
|||
|
eGFR (ml/min/1.73m2) |
99.6 ± 26.1 |
108.7 ± 33.8 |
91.3 ± 13.1 |
|||
|
Ferritin (ng/mL) |
176 ± 242 |
249 ± 336 |
109 ± 76 |
|||
|
Total cholesterol (mg/dL) |
217 ± 41 |
212 ± 41 |
221 ± 42 |
|||
|
LDL cholesterol (mg/dL) |
126 ± 39 |
115 ± 44 |
138 ± 30 |
|||
|
HDL cholesterol (mg/dL) |
70.7 ± 20.7 |
76.5 ± 23.2 |
64.2 ± 16.3 |
|||
|
LDL/HDL ratio |
1.94 ± 0.82 |
1.62 ± 0.71 |
2.30 ± 0.83 † |
|||
|
Triglyceride (mg/dL) |
122 ± 84 |
120 ± 100 |
125 ± 66 |
|||
|
Fasting blood glucose (mg/dL) |
104 ± 14 |
107 ± 18 |
101 ± 9 |
|||
|
Fasting insulin (μIU/mL) |
5.49 ± 3.75 |
4.58 ± 3.06 |
6.32 ± 4.25 |
|||
|
HOMA-R |
1.45 ± 1.07 |
1.29 ± 1.09 |
1.59 ± 1.09 |
Table 1: Baseline characteristics of participants
SN13T, lactobacillus plantarum SN13T; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA-R, homeostasis model assessment ratio
MEAN ± SD (all such values).
† P<0.05 versus the heat-killed SN13T group.
The compliance with taking 120 mL/day of the SN13T beverage throughout the 16-week intake period was 97.7 ± 4.1% in the heat-killed SN13T group and 98.2 ± 2.6% in the live SN13T group according to the daily records. No significant difference in compliance was observed between the intervention groups. The changes in calorie intake were also not significantly different, -235 ± 530 kcal and 24 ± 487 kcal in the heat-killed group and the live SN13T one, respectively.
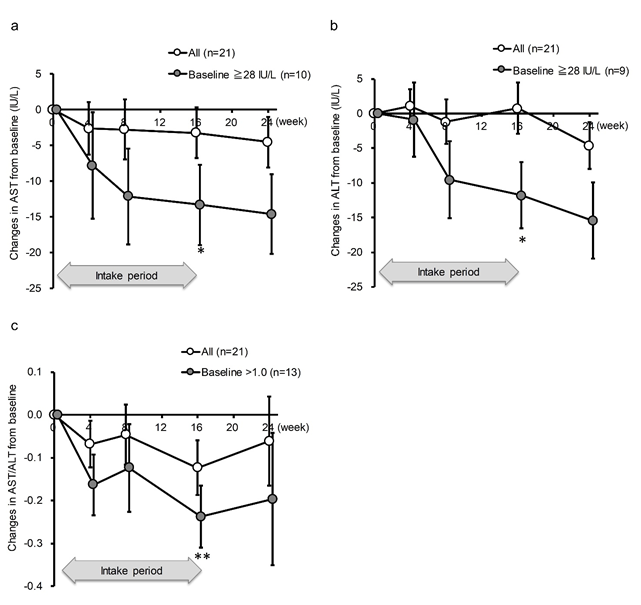
AST and ALT were not significantly decreased by the intake of SN13T in either group, as determined by a paired-t test within each group (Table 2). However, when subgroup of the AST >= 28 U/L, whichever the intervention group, was analyzed, AST was significantly decreased at an average of 13.3 U/L (22.6 ± 20.6% reduction from baseline, P=0.042) in the AST >=28 U/L subgroup (Fig. 2a). The same phenomenon was observed for ALT at the same threshold (26.6 ± 23.7% reduction from baseline, P=0.039, Fig. 2b). The effect size between the subgroups were large in both AST and ALT (1.45 and 1.67, respectively). Furthermore, even after terminating the intake of SN13T at week 16, the values of AST and ALT continued to decrease until week 24 in higher baseline subgroups (Fig. 2a and 2b). Although the AST/ALT ratio did not change in either group (Table 2), the subgroup analysis with AST/ALT>1 at baseline showed the significant reduction in the AST/ALT ratio. In contrast with AST, ALT, or the AST/ALT ratio, this tendency was not seen in γ-GTP or alkaline phosphatase, lacking obvious threshold (Table 3).
Figure 2: AST and ALT in the subgroups with >=28 U/L and AST/ALT ratio with >1.0 at baseline were decreased by SN13T intake. The changes in (a) AST, (b) ALT, or (c) AST/ALT ratio over time. *P<0.05 and ** P<0.01 versus baseline (0w) by paired t-test. Included numbers of subjects for subgroup analyses are 10 for AST >=28 U/L, 9 for ALT >=28 U/L, 13 for AST/ALT ratio >1.0, and 8 for AST/ALT ratio<=1.0.
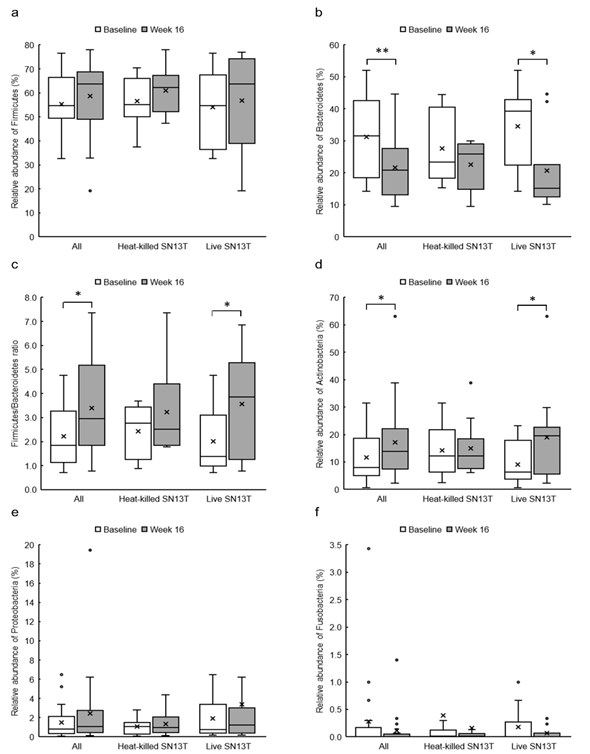
Figure 3: The changes of relative abundances of predominant fecal bacterial phyla and Firmicutes/Bacteroidetes ratio in all subjects (n=21), the heat-killed SN13T group (n=10), and the live SN13T group (n=11). (a) Firmicutes, (b) Bacteroidetes, (c) Firmicutes/Bacteroidetes ratio, (d) Actinobacteria, (e) Proteobacteria, and (f) Fusobacteria.
*P<0.05 and ** P<0.01 versus corresponding baseline (0w) by paired t-test.
|
Baseline |
Week 16 |
Week 24 |
Changes in 16 weeks |
Changes in 24 weeks |
|
|
AST (U/L) |
|||||
|
Heat-killed SN13T |
35.0 ± 25.1 |
30.3 ± 8.3 |
27.8 ± 8.1 |
-4.7 ± 22.3 |
-7.2 ± 21.3 |
|
Live SN13T |
29.9 ± 12.3 |
28.0 ± 5.6 |
27.8 ± 6.2 |
-1.9 ± 8.8 |
-2.1 ± 9.8 |
|
ALT (U/L) |
|||||
|
Heat-killed SN13T |
24.5 ± 10.9 |
27.4 ± 13.6 |
22.2 ± 6.6 |
2.9 ± 18.0 |
-2.3 ± 8.9 |
|
Live SN13T |
39.7 ± 30.5 |
38.5 ± 26.5 |
33.0 ± 17.6 |
-1.2 ± 16.5 |
-6.7 ± 19.9 |
|
AST/ALT |
|||||
|
Heat-killed SN13T |
1.40 ± 0.63 |
1.23 ± 0.42 |
1.27 ± 0.23 |
-0.17 ± 0.31 |
-0.13 ± 0.60 |
|
Live SN13T |
1.00 ± 0.39 |
0.92 ± 0.34 |
1.00 ± 0.37 |
-0.08 ± 0.28 |
0.00 ± 0.34 |
|
γ-GTP (U/L) |
|||||
|
Heat-killed SN13T |
67.8 ± 34.8 |
93.6 ± 71.6 |
74.4 ± 58.3 |
25.8 ± 59.2 |
6.6 ± 35.2 |
|
Live SN13T |
77.3 ± 43.2 |
81.9 ± 55.3 |
85.2 ± 65.6 |
4.6 ± 37.7 |
7.9 ± 49.8 |
|
Alkaline phosphatase (U/L) |
|||||
|
Heat-killed SN13T |
204 ± 60 |
247 ± 65 |
230 ± 90 |
42 ± 56 |
25 ± 49 |
|
Live SN13T |
232 ± 64 |
244 ± 59 |
238 ± 59 |
11 ± 25 |
6 ± 26 |
|
Total cholesterol (mg/dL) |
|||||
|
Heat-killed SN13T |
212 ± 41 |
226 ± 51 |
227 ± 34 |
14 ± 22 |
15 ± 22 |
|
Live SN13T |
221 ± 42 |
226 ± 36 |
223 ± 48 |
5 ± 11 |
1 ± 12 |
|
HDL cholesterol (mg/dL) |
|||||
|
Heat-killed SN13T |
78.5 ± 23.5 |
81.2 ± 29.6 |
82.8 ± 31.5 |
2.7 ± 12.9 |
4.3 ± 13.6 |
|
Live SN13T |
63.5 ± 15.7 |
66.5 ± 13.2 |
64.2 ± 11.2 |
2.9 ± 5.9 |
0.6 ± 5.5 |
|
LDL cholesterol (mg/dL) |
|||||
|
Heat-killed SN13T |
113 ± 46 |
121 ± 48 |
117 ± 35 |
7 ± 23 |
4 ± 22 |
|
Live SN13T |
137 ± 29 |
138 ± 24 |
134 ± 30 |
1 ± 11 |
-3 ± 12 |
|
LDL/HDL ratio |
|||||
|
Heat-killed SN13T |
1.56 ± 0.71 |
1.63 ± 0.76 |
1.60 ± 0.70 |
0.07 ± 0.21 |
0.04 ± 0.37 |
|
Live SN13T |
2.29 ± 0.79 |
2.19 ± 0.71 |
2.14 ± 0.67 |
-0.11 ± 0.19 |
-0.15 ± 0.34 |
|
Triglyceride (mg/dL) |
|||||
|
Heat-killed SN13T |
122 ± 105 |
128 ± 51 |
128 ± 100 |
6 ± 85 |
7 ± 60 |
|
Live SN13T |
122 ± 63 |
121 ± 81 |
144 ± 111 |
-1 ± 38 |
21 ± 61 |
|
Fasting blood glucose (mg/dL) |
|||||
|
Heat-killed SN13T |
107.9 ± 18.6 |
107.5 ± 16.1 |
106.5 ± 12.2 |
-0.4 ± 7.4 |
-1.4 ± 14.5 |
|
Live SN13T |
101.1 ± 8.4 |
102.5 ± 15.1 |
101.4 ± 9.7 |
1.5 ± 8.3 |
0.3 ± 5.0 |
|
Fasting insulin (μIU/mL) |
|||||
|
Heat-killed SN13T |
4.58 ± 3.06 |
4.96 ± 2.35 |
4.98 ± 2.50 |
0.38 ± 3.12 |
0.40 ± 3.43 |
|
Live SN13T |
6.32 ± 4.25 |
6.99 ± 3.72 |
5.68 ± 2.72 |
0.67 ± 1.56 |
-0.64 ± 2.97 |
|
HOMA-R |
|||||
|
Heat-killed SN13T |
1.29 ± 1.09 |
1.28 ± 0.53 |
1.30 ± 0.67 |
-0.01 ± 0.96 |
0.01 ± 1.25 |
|
Live SN13T |
1.59 ± 1.09 |
1.78 ± 0.92 |
1.42 ± 0.67 |
0.19 ± 0.44 |
-0.17 ± 0.78 |
|
Serum TNF-α (pg/ml) |
|||||
|
Heat-killed SN13T |
3.30 ± 0.42 |
2.96 ± 0.34 |
- |
-0.34 ± 0.49 |
- |
|
Live SN13T |
3.35 ± 0.51 |
3.13 ± 0.31 |
- |
-0.22 ± 0.36 |
- |
Table 2: Liver enzymes and other serum biochemical variables at pre-and post-intervention period
SN13T, lactobacillus plantarum SN13T; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA-R, homeostasis model assessment ratio; TNF-α: tumor necrosis factor-α; MEAN ± SD (all such values).
Heat-killed SN13T group (n=10), Live SN13T (n=11).
|
Baseline |
Week 16 |
Week 24 |
Changes |
Changes |
|
|
γ-GTP (U/L) |
72.8 ± 38.8 |
87.5 ± 62.2 |
80.0 ± 60.9 |
14.7 ± 49.0 |
7.3 ± 42.4 |
|
Alkaline phosphatase (U/L) |
219 ± 62 |
245 ± 60* |
234 ± 74 |
26 ± 44 |
15 ± 39 |
|
Serum TNF-α (pg/mL) |
3.33 ± 0.46 |
3.05 ± 0.33** |
- |
-0.28 ± 0.42 |
- |
Table 3: Whole-group analyses of γ-GTP, alkaline phosphatase, and TNF-α
γ-GTP, γ-glutamyl transpeptidase; TNF-α: tumor necrosis factor-α; MEAN ± SD (all such values).
*P<0.05 and ** P<0.01 versus baseline (n=21).
Other outcomes, total cholesterol, HDL cholesterol, LDL cholesterol, LDL/HDL ratio, triglycerides, fasting blood glucose, fasting insulin, HOMA-R, and TNF-α, did not differ between the intervention groups. However, both the live and heat-killed SN13T groups showed a tendency of serum TNF-α reduction, at P=0.054 and P=0.069, respectively. When serum TNF-α was analyzed in all subjects together, a significant decrease was observed (P=0.006) at week 16 (Table 3).
The bowel movement frequencies were unchanged in both groups (data now shown).
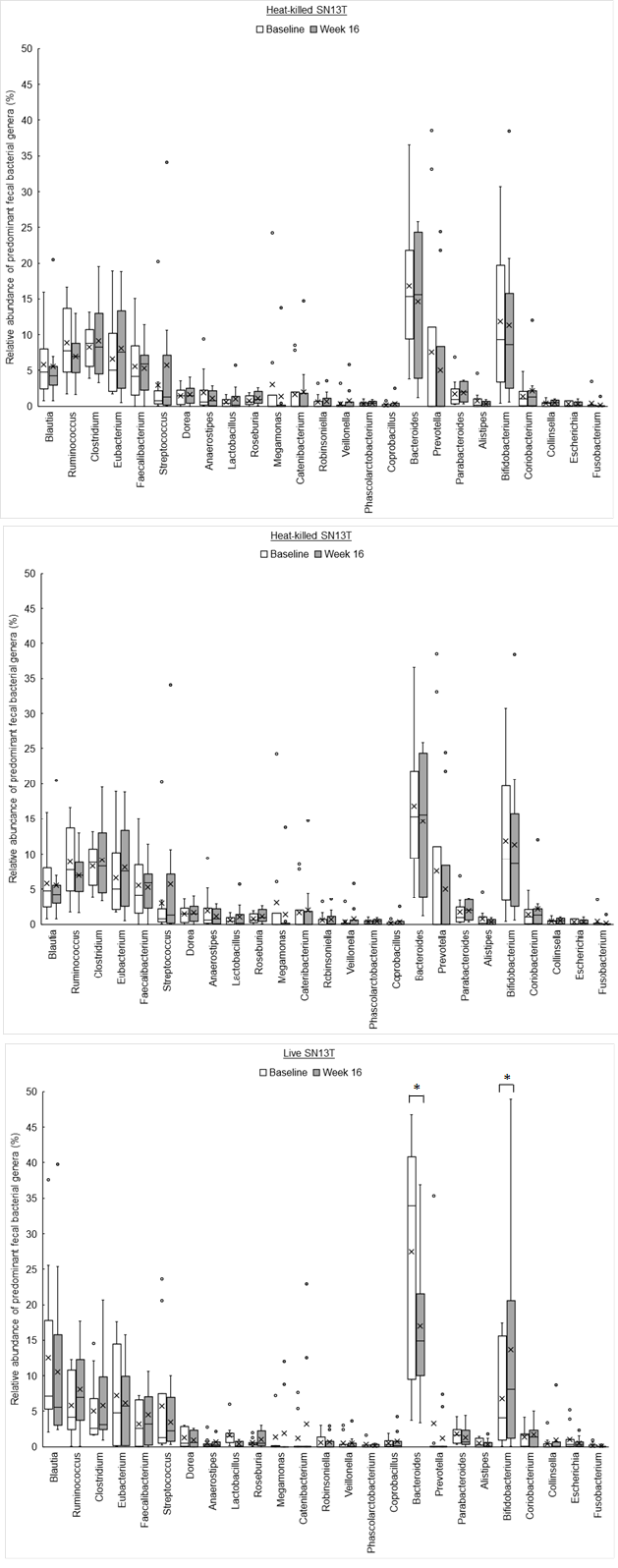
The phylum level analyses for fecal microbiota revealed that a relative abundance of Firmicutes tended to increase, no matter which group, with the consumption of SN13T for 16 weeks (Fig. 3a). On the other hand, a decreasing tendency and a significant decrease (P=0.014) of Bacteroidetes in the heat-killed SN13T group and live SN13T group were observed, respectively (Figure 3b, all subjects: P=0.006 vs. baseline). As a corollary, the Firmicutes/Bacteroidetes ratio showed an increasing tendency (Figure 3c, all subjects: P=0.015 and the live SN13T group: P=0.043 vs. baseline). Actinobacteria was significantly elevated in the live SN13T group, but not in the heat-killed SN13T group (Figure 3d, P=0.023 vs. baseline). Proteobacteria did not change significantly in either group (Figure 3e). The relative abundance of Fusobacteria tended to decline in both groups (Figure 3f). These top five phyla, namely Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Fusobacteria, occupied 99.97% of the total bacterial count in feces. The changes of predominant genera can be found as Supplementary Figure 1S online.
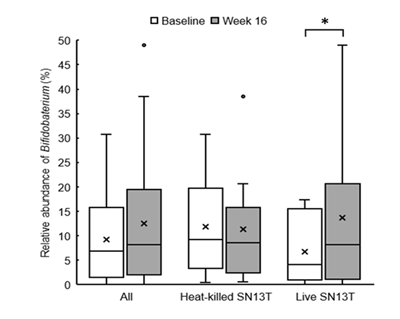
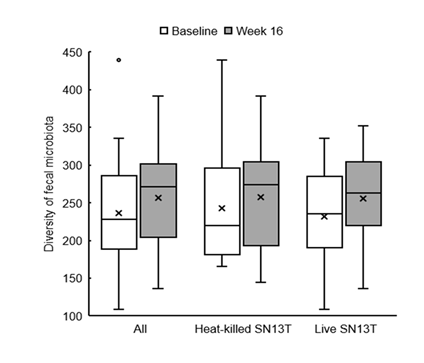
Bifidobacterium accounted for most of the elevation of Actinobacteria (Figure 4, P=0.044 vs. baseline). Furthermore, the microbiota diversity was evaluated by means of the Chao1 estimator, and it tended to increase in both intervention groups (Figure 5).
In the assessment of safety outcomes, the body-fat percentage and systolic blood pressure became higher than the baselines in both groups during the intake period. No significant difference was observed between the groups. Other clinical tests and the subjective symptom did not show any abnormal change.
*P<0.05 versus baseline (0w) by paired t-test.
Discussion
Alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) are common cause of liver disease. Although there is no targeting therapy for ALD and NAFLD, various approaches including probiotics have been investigated [4]. The combined consumption of Lb. delbrueckii. subsp. bulgaricus and Streptococcus thermophiles reduced AST, ALT, and γ-GTP within the group comparison in subjects with NAFLD confirmed by liver biopsy [17]. Bifidobacterium longum with fructooligosaccharides and lifestyle modification significantly decreased AST and TNF-α levels when compared with lifestyle modification alone in subjects with NASH confirmed by liver biopsy [18]. The importance of gut microbiota is implied by the fact that intestinal permeability in NAFLD was elevated and that intestinal permeability and the prevalence of small intestinal bacterial overgrowth were correlated with the severity of steatosis [19].
In this pilot clinical study, we evaluated the effect of Lb. plantarum SN13T on liver function in subjects with mild hepatic dysfunction. We have previously observed that the continuous consumption of yogurt made with SN13T reduced γ-GTP, AST, and ALT in a subgroup with mild hepatic dysfunction out of those with gastrointestinal complaints [8]. The previous study had not been assigned to analyze hepatic function, and most of subjects showed normal hepatic function. Therefore, to confirm the effect on hepatic function, previously observed in the subgroup analysis in a clinical study with different aim, in subjects with hepatic dysfunction, the present pilot study was planned on a small scale.
One criterion for inclusion in this study was that any of AST, ALT, or γ-GTP value was above a certain level; accordingly, subjects with a normal range of these parameters were included.
AST and ALT were significantly reduced by the consumption of SN13T in subjects whose baseline were not less than 28 U/L, regardless of whether the SN13T cells were alive or dead. This threshold, 28 U/L, was established as a clear bifurcation; that is, the upper category included 9 decreases per 10 in AST and 8 decreases per 9 in ALT, whereas the lower category included 1 decrease per 11 in AST and 0 decreases per 12 in ALT.
Different from AST, ALT or the AST/ALT ratio, an obvious borderline or significant decrease, which was observed in the previous clinical trial, was not observed in γ-GTP. In the case of alkaline phosphatase, most subjects except for one had the values within the standard range at baseline. Therefore, it was no wonder that alkaline phosphatase didn’t alter by the SN13T administration.
In the current study, the SN13T cells were taken as carrot juice instead of yogurt as in the previous study. The heat-killed SN13T juice was made by heating after fermentation; accordingly, both beverages contained somewhat functional substances produced by SN13T during fermentation. Therefore, the reason why AST and ALT reduction were observed with both live and dead SN13T cells might be that thermally stable substances contained in the beverage improved liver function. The inconsistency between the current and previous clinical studies might derive from the different fermentation processes in diverse fermented media, resulting in the production of altered secretion materials. Moreover, in our animal study, the oral administration of the live SN13T cells, but not the heat-killed cells, rescued alcohol poisoning mice [20], implying the species difference. Interestingly, the reduction in AST and ALT not only lasted after the cessation of beverage consumption but also continued to be at a much greater degree until the final clinical visit, at 24 weeks, which is 8 weeks after the final consumption. This suggests that the SN13T cells brought long-lasting hepatic improvement.
TNF-α is a proinflammatory cytokine, and the serum TNF-α level was elevated in patients with NASH [21]. TNF-α mRNA expression levels were also enhanced in the adipose tissue and/or liver, and those levels were associated with the severity of NAFLD or NASH [22-24]. Moreover, it has been shown that obesity-related insulin resistance was related to TNF-α expression levels in adipose tissue [24]. In this study, no difference was observed in the level of serum TNF-α between the live and heat-killed SN13T groups. However, the serum TNF-α concentration showed a significant reduction after 16 weeks of SN13T consumption when analyzed in all subjects, implying the possibility of a decline in systemic inflammation.
In the current study, we analyzed the change in stool frequency, since SN13T is a probiotic expected to regulate bowel conditions according to the previous study [8]. However, no gastrointestinal improvements were observed in the current study, since the criteria did not include gastrointestinal problems but rather mild hepatic dysfunction, then the subjects originally showed normal bowel movements.
Interestingly, the changes in intestinal flora were shown by the metagenome analyses of the fecal microbiota. As mentioned above, the overgrowth of intestinal bacteria and the enhanced intestinal permeability may relate to NAFLD and NASH [19, 21]. Another research group has shown that the intake of Bifidobacterium?longum is effective to improve hepatic function [12]. Therefore, the increase of Bifidobacterium cells in the colon could partly explain why AST and ALT were reduced. However, in the present study, the reductions in AST and ALT were observed in both experimental groups, whereas only the live SN13T group showed the increase of Bifidobacterium in the gut microbiota. There is also a possibility that the improvement of intestinal permeability reduces LPS leaking into the blood flow, resulting in TNF-α reduction.
In obese people, the proportion of Firmicutes in the intestinal microbiome tended to be predominant, whereas that of Bacteroidetes is conversely predominant in lean people, resulting in a high Firmicutes to Bacteroidetes (F/B) ratio in obese people and a low one in lean people [25]. In this study, the subjects were not obese. The body fat rather increased through the clinical trial without contradicting to that F/B ratio was increased. In this context, it is obvious that hepatic function was not improved due to the remediation of obesity.
Interestingly, the decrease in Fusobacteria and the increase in fecal microbiota diversity were also observed in both experimental groups. Although only 6 (3 for each intervention group) out of 21 subjects were detected over 0.05% of Fusobacteria (Fusobacterium) abundance at baseline, the administration of SN13T cells reduced all cases by more than 50% (71% as the average). It has been recently reported that Fusobacterium is associated with colorectal cancer [26, 27] and inflammatory bowel disease [28, 29]. Moreover, there are reports that gut dysbiosis is related to various common diseases [5-7]. In these respects, the plant-derived Lb. plantarum SN13T may have possible therapeutic implications for not only hepatic dysfunction but also gut microbiota–related diseases. It is currently uncertain whether there is the direct relationship between the gut microbiota alteration and the improvement of hepatic dysfunction by the intake of SN13T. The study to elucidate the question is in progress.
In conclusion, the administration of plant-derived Lb. plantarum SN13T is a useful and harmless strategy to improve mild liver dysfunction, for which no targeted therapy currently exists, possibly via modulation of the gut microbiota.
Acknowledgments
We thank Nomura dairy Products Co., Ltd to produce the Lb. plantarum SN13T-fermented- beverages used for this study.
Author contributions
FH and MS designed the research and had primary responsibility for the final content. FH, DN, and KK, RI and ST have conducted the research. FH performed the statistical analysis and wrote the paper. All authors have read and approved the final manuscript.
Conflict of interest
Hiroshima University has a patent with respect to the use of Lb. plantarum SN13T. Professor Masanori Sugiyama is the inventor on the patent. The remaining authors have no conflict of interest to declare.
References
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 313 (2015): 2263-73.
- Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, Nguyen MH. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One 12 (2017): e0173499.
- Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 4 (2019): 389-98.
- Singh S, Osna NA, Kharbanda KK. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J Gastroenterol 23 (2017): 6549-70.
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms (2019): 7.
- Belizário JE, Faintuch J, Garay-Malpartida M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediators Inflamm (2018): 2037838.
- Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One 10 (2015): e0137429.
- Higashikawa F, Noda M, Awaya T, Nomura K, Oku H, Sugiyama M. Improvement of constipation and liver function by plant-derived lactic acid bacteria: a double-blind, randomized trial. Nutrition 26 (2010): 367-74.
- Górska, A., Przystupski, D., Niemczura, M. J. & Kulbacka, J. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr Microbiol 76 (2019): 939-49.
- Higashikawa F, Noda M, Awaya T, Danshiitsoodol N, Matoba Y, Kumagai T, Sugiyama M. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: a randomized, double-blind, placebo-controlled clinical trial. Eur J Clin Nutr 70 (2016): 582-7.
- Saez-Lara MJ, Gomez-Llorente C, Plaza-Diaz J, Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. Biomed Res Int (2015): 505878.
- Wu Y, Zhang Q, Ren Y, Ruan Z. Effect of probiotic Lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PLoS One 12 (2017): e0178868.
- Wang L, Zhang J, Guo Z, Kwok L, Ma C, Zhang W, Lv Q, Huang W, Zhang H. Effect of oral consumption of probiotic Lactobacillus planatarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition 30 (2014): 776-83.
- Park S, Kang J, Choi S, Park H, Hwang E, Kang YG, Kim AR, Holzapfel W, Ji Y. Cholesterol-lowering effect of Lactobacillus rhamnosus BFE5264 and its influence on the gut microbiome and propionate level in a murine model. PLoS One 13 (2018): e0203150.
- Ueno M, Kikuchi M, Oshima K, Kim SW, Morita H, Hattori M. Assessment and Improvement of Methods for Microbial DNA Preparation from Fecal Samples. Handbook of Molecular Microbial Ecology II: Metagenomics in Different Habitats (ed. by Frans J. de Bruijn) (2011): 191-8.
- Kim SW, Suda W, Kim S, Oshima K, Fukuda S, Ohno H, Morita H, Hattori M. Robustness of gut microbiota of healthy adults in response to probiotic intervention revealed by high-throughput pyrosequencing. DNA Res 20 (2013): 241-53.
- Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci 15 (2011): 1090-5.
- Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci 57 (2012): 545-53.
- Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 49 (2009): 1877-87.
- Noda M, Maruyama M, Danshiitsoodol N, Higashikawa F, Sugiyama M. Improvement of Alcohol-Poisoning Symptoms in Mice by the Oral Administration of Live Lactobacillus plantarum SN13T Cells. Int J Mol Sci 21 (2020): 1896.
- Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 48 (2001): 206-11.
- Jorge ASB, Andrade JMO, Paraíso AF, Jorge GCB, Silveira CM, de Souza LR, Santos EP, Guimaraes ALS, Santos SHS, De-Paula AMB. Body mass index and the visceral adipose tissue expression of IL-6 and TNF-alpha are associated with the morphological severity of non-alcoholic fatty liver disease in individuals with class III obesity. Obes Res Clin Pract 12 (2018): 1-8.
- Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 34 (2001): 1158-63.
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95 (1995): 2409-15.
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 444 (2006): 1022-3.
- Amitay EL, Werner S, Vital M, Pieper DH, Höfler D, Gierse IJ, Butt J, Balavarca Y, Cuk K, Brenner H. Fusobacterium and colorectal cancer: causal factor or passenger? Results from a large colorectal cancer screening study. Carcinogenesis 38 (2017): 781-8.
- Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358 (2017): 1443-8.
- Minami M, Ando T, Okamoto A, Sasaki N, Ohkura T, Torii K, Hasegawa T, Ohta M, Goto H. Seroprevalence of Fusobacterium varium in ulcerative colitis patients in Japan. FEMS Immunol Med Microbiol 56 (2009): 67-72.
- Ohkusa T, Sato N, Ogihara T, Morita K, Ogawa M, Okayasu I. Fusobacterium varium localized in the colonic mucosa of patients with ulcerative colitis stimulates species-specific antibody. J Gastroenterol Hepatol 17 (2002): 849-53.