Joint Pathologies as a Covert Risk Factor for Rod Fracture after Pedicle Subtraction Osteotomy in Adult Spinal Deformity
Article Information
Ki Young Lee, Jung-Hee Lee*, Sang-Kyu Im
Department of Orthopedic Surgery, Graduate School, College of Medicine, Kyung Hee University, Seoul, Korea
*Corresponding author: Jung-Hee Lee, Department of Orthopedic Surgery, Graduate School, College of Medicine, Kyung Hee University, Kyungheedae-ro, Dongdaemun-gu, Seoul, 130-872, Korea.
Received: 16 November 2021; Accepted: 29 November 2021; Published: 06 December 2021
Citation:
Lee KY, Lee JH, Im SK. Joint Pathologies as a Covert Risk Factor for Rod Fracture after Pedicle Subtraction Osteotomy in Adult Spinal Deformity. Archives of Clinical and Biomedical Research 5 (2021): 940-958.
View / Download Pdf Share at FacebookAbstract
Background: The recent increase in average life expectancy has increased the prevalence of adult spinal deformity (ASD). Instrumentation failure, including rod fracture (RF), may occur even after successful procedures. RF is the most common cause of revision surgery and pedicle subtraction osteotomy (PSO) is a major risk factor of RF. However, disagreements remain regarding how results are related to hip and knee joint pathologies.
Methods: Participants comprised 89 consecutive patients (mean age, 71.2 y) who underwent deformity correction including PSO, with ≥2 years of follow-up. Patients were classified into non-RF (n = 48) and RF groups (n = 41). Radiologic factors including spinopelvic and lower-extremity parameters were measured.
Results: Both groups showed severe sagittal imbalance preoperatively. There were no significant differences between groups in patient factors, sagittal and coronal spinopelvic parameters, and osteoarthritis (OA) grade of the joints. However, preoperative structural and functional leg length discrepancy (LLD) and pelvic obliquity significantly differed between groups (P = 0.001, 0.002, and 0.002, respectively). The proportion of knee OA, structural and functional LLD, and knee angular deformity were significantly higher in the RF group than in the non-RF group (P = 0.008, 0.000, 0.020, and 0.012, respectively).
Conclusion: Elderly patients with ASD often show degenerative changes in the lower extremities, and even if spine deformity correction is successfully performed, complications, including RF, can occur if joint pathologies are not resolved. Therefore, it is important to consider perioperative treatments for the lower extremities as well as restoration of the spine and pelvis.
Keywords
Adult spinal deformity; Elderly; Hip joint; Knee joint; Knee angular deformity; Leg length discrepancy; Osteoarthritis; Pedicle subtraction osteotomy; Rod fracture; Spine
Article Details
1. Introduction
The recent increase in life expectancy and active senior lifestyle has increased the demand for the treatment of age-related disabilities. Adult Spinal Deformity (ASD) is a representative age-related disability of the spine, and interest in surgical deformity correction of ASD that recovers and maintains the normal upright posture required for basic human needs has increased [1]. Of the surgical options for ASD, Pedicle Subtraction Osteotomy (PSO) is one of the most powerful methods for achieving ideal Lumbar Lordosis (LL) correction and optimal sagittal balance [2].
However, problems of PSO remain, originating not only from the complexity of the procedure itself, but also from the reported complications [3,4]. In particular, instrumentation failure, including rod fracture (RF), is the most common cause of revision surgery, and RF can be caused by PSO and other risk factors [5]. Therefore, several instruments and surgical techniques have been suggested to decrease RF [6], however, age should not be overlooked for the deformity correction in patients with ASD [7].
Most patients with ASD undergoing deformity correction are elderly, and often have comorbid degenerative joint diseases, such as hip and knee osteoarthritis (OA) [8,9]. However, it is unclear whether lower-extremity joint pathologies affect spinal disease or vice versa. In addition, there is a lack of studies on the effects of joint pathologies in patients with ASD who underwent long-segment fixation. Therefore, this study assessed the relationship between structural and biochemical changes caused by lower-extremity joint pathologies and RFs after long-segment fixation including PSO in patients with ASD aged >65 years.
2. Material and Methods
2.1 Patient selection
This study was approved by the ethics committee of our institution. We retrospectively reviewed 130 consecutive patients with ASD treated between 2009 and 2018. The inclusion criteria were as follows: age ≥65 years; ASD accompanied by sagittal malalignment (sagittal vertical axis [SVA] >50 mm, pelvic incidence [PI] minus LL >10°, and pelvic tilt [PT] >25°); ≥2 years of follow-up after deformity correction; surgically treated with long-segment fixation with sacropelvic fixation, with the uppermost and lowermost instrumented vertebrae set at the T10 and S1 levels, respectively; clear atrophy of the back musculature on cross-sectional magnetic resonance imaging and computed tomography (CT) images as a diagnostic criterion for lumbar degenerative kyphosis (LDK) and clinical signs such as walking difficulties with stooping, inability to lift heavy objects toward the front, difficulty in climbing slopes, and the need for elbow support when working in the kitchen, resulting in a hard corn on the extensor surface of the elbow [10-12]. The diagnosis of RF was based on rod breakage with recent fusion mass fracture, observed on plain radiography and CT, confirmed by uptake in either bone scan or SPECT images [5]. Patients were classified into RF and non-RF groups.
2.2 Surgical method
Every patient underwent PSO at L2 or L3, including closing-opening wedge osteotomy; the anterior vertebral cortex was fractured in patients requiring more correction [13]. The lamina at the PSO site was preserved and used for the fusion bed in cases without spinal stenosis at the PSO site by approximating the remaining upper and lower laminae of the proximal and osteotomized vertebrae, respectively. However, posterior decompression with posterolateral fusion was performed by removing the remaining laminae in cases with spinal stenosis at the PSO site. A morselized autograft was used for fusion, with a mixture of demineralized bone matrix and chipped-bone allograft [5].
2.3 Radiographic measurements
Coronal and sagittal alignments were evaluated using anteroposterior and lateral 14×36-inch whole-spine radiographs. Sagittal spine radiographs were obtained with the patient standing in a neutral unsupported fists-on-clavicle position [14]. All digital radiographs were reviewed preoperatively, and at 2 months (i.e. postoperatively) and 2 years after surgery (i.e. last follow-up), using validated software (Surgimap, Nemaris Inc.) [15]. And the last follow-up radiographic parameters of the RF group were measured at the last visit to the outpatient clinic before the onset of RF.
As sagittal spinal parameters, we measured the PI, sacral slope (SS), PT, thoracic kyphosis (TK, T5-12), thoracolumbar junction (TL, T10-L2), LL (T12-L2), lumbosacral junction (LS, T12-S1), and SVA [16,17]. As coronal spinal parameters, we measured the coronal Cobb angle (for patients with multiple coronal curvatures, the largest angle was evaluated) and coronal C7 plumb line minus the central sacral vertical line (coronal C7PL).
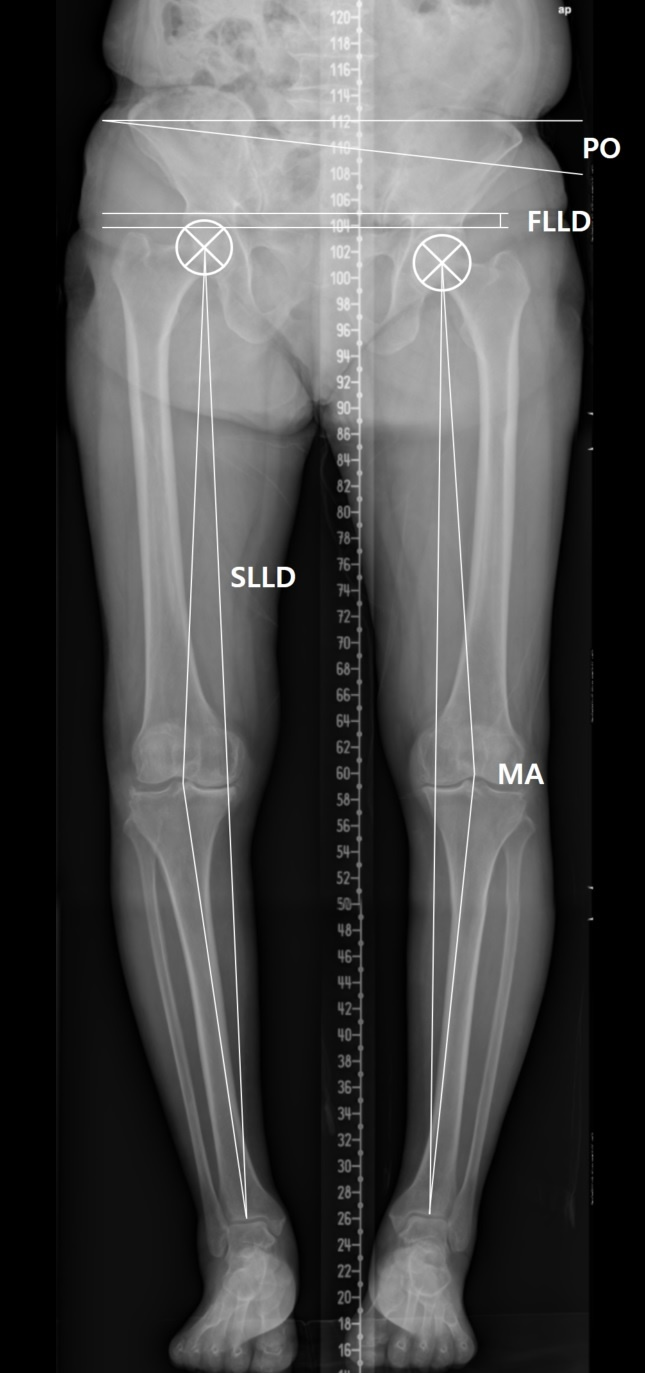
Orthogram was used to evaluate hip and knee joint pathologies (Figure 1). Kellgren-Lawrence grades 3 and 4 were considered to indicate pathology [18]. Definitions of clinically significant Leg Length Discrepancy (LLD) vary; we considered 10 mm to represent clinically significant LLD in the present study [19]. The functional LLD was defined as the height difference between right and left femoral horizontal reference lines (defined as the horizontal line tangent to the uppermost part of the femoral head) [20]. The structural LLD was defined as the distance between the center of the femoral head and the midpoint of the tibial plafond [21]. Pelvic Obliquity (PO) was defined as the angle between the horizontal reference line and pelvic coronal reference line (measured between the tips of the sacral ala) [20]. The angles of the line from the femoral head center to the articulating center of the distal femur at the knee and the line from the articulating center of the proximal tibia at the knee to the tibial plafond midpoint were measured to assess the angular deformity of the knee (mechanical axis) [22]. A varus or valgus angle ≥10° in the measured mechanical axis (negative for varus and positive for valgus) was defined as an angular deformity.
Figure 1: Orthogram was used to evaluate hip and knee joint pathologies. The functional LLD (FLLD) was defined as the height difference between right and left femoral horizontal reference lines. The structural LLD (SLLD) was defined as the distance between the center of the femoral head and the midpoint of the tibial plafond. Pelvic Obliquity (PO) was defined as the angle between the horizontal reference line and pelvic coronal reference line. The angles of the line from the femoral head center to the articulating center of the distal femur at the knee and the line from the articulating center of the proximal tibia at the knee to the tibial plafond midpoint were measured to assess the Mechanical Axis (MA).
2.4 Clinical outcome assessment
Clinical outcomes were assessed using the Oswestry Disability Index (ODI) and Visual Analog Scale (VAS) preoperatively, postoperatively, and at the last follow-up.
2.5 Statistical analysis
Statistical analysis was performed using SPSS software (version 20.0; SPSS Inc., Chicago, IL, USA). Continuous variables were evaluated by analyses of variance, unpaired t-tests, and the Wilcoxon's rank sum test, as appropriate. Categorical variables were assessed using the chi-square test and Fisher’s exact test, as appropriate. The relationship between radiographic parameters was evaluated by the Pearson’s correlation coefficient, and a multivariate logistic regression analysis (backward elimination method) was performed to identify the risk factors of RF. Statistical significance was set at P < 0.05.
3. Results
3.1 Baseline patient characteristics
Of 130 patients screened, 89 (2 men and 87 women; mean age at surgery, 71.2 years; mean follow-up period, 63.4 months) met the inclusion criteria. The mean Body Mass Index (BMI) was 25.1 kg/m2 and mean bone mineral density (BMD) was 0.96 gm/cm2.
3.2 RF characteristics
RF occurred in 41 patients at a mean of 23 months (20 and 23 months for 17 bilateral and 24 unilateral cases, respectively). RF was found at the PSO site in 37 patients and at L4-5 in 4 patients. Most patients complained of sudden back pain with a cracking noise in the back due to bending over or accidently falling, and underwent revision surgery to reduce back pain and prevent further sagittal imbalance. However, in one patient, unilateral non-symptomatic RF refused revision surgery and underwent close observation.
3.3 Sagittal spinopelvic radiographic parameters (Table 1)
Preoperatively, the patients showed severe sagittal imbalance in both groups, without significant differences. Postoperatively, the SVA, TK, LL, and TK were improved in both groups, without significant differences. At last follow-up, the SVA indicated well-maintained sagittal balance, and the TK, LL, and PT indicated spinopelvic harmony, in both groups, without significant differences.
3.4 Coronal spinal radiographic parameters (Table 1)
Preoperatively, the coronal Cobb angle and pre-coronal C7PL did not significantly differ between groups. After deformity correction, the coronal Cobb angle was normalized, and as with the C7PL, did not show a significant difference between groups (postoperatively and at the last follow-up).
3.5 Preoperative lower-extremity radiographic parameters (Table 1)
Preoperatively, the structural and functional LLD and PO were significantly greater in the RF group and in the non-RF group (P < 0.05). However, the right and left mechanical axes and lower-extremity OA grades were not significantly different between the two groups.
|
Variables |
Non-RF group(n=48) |
RF group(n=41) |
P-value |
|
Mean ±SD |
Mean ±SD |
||
|
Patient factors |
|||
|
Age ( year) |
71 ± 5.6 |
71.4 ± 4.9 |
0.701 |
|
BMD T-score (gm/cm2) |
-1.34 ± 0.93 |
-1.81 ± 1.3 |
0.050 |
|
BMD (gm/cm2) |
0.98 ± 0.12 |
0.93 ± 0.16 |
0.065 |
|
BMI (kg/m2) |
25.4 ± 3.2 |
24.9 ± 3.6 |
0.454 |
|
Sagittal parameters |
|||
|
Pre SVA (mm) |
189.8 ± 70.7 |
201.3 ± 91 |
0.503 |
|
Post SVA (mm) |
-9.4 ± 28.1 |
-19.1 ± 18.9 |
0.066 |
|
SVA correction (mm) |
-199.2 ± 82.6 |
-220.3 ± 89.5 |
0.250 |
|
Last SVA (mm) |
12.7 ± 36.5 |
12.9 ± 27.8 |
0.975 |
|
Pre TK (°) |
4.2 ± 15.4 |
-1.3 ± 12.5 |
0.069 |
|
Post TK (°) |
28.6 ± 12.5 |
24.5 ± 9.2 |
0.075 |
|
TK correction (°) |
24.4 ± 10.1 |
25.8 ± 11.7 |
0.550 |
|
Last TK (°) |
35.7 ± 15 |
31.6 ± 14.5 |
0.195 |
|
Pre TL (°) |
7.6 ± 17.1 |
7 ± 17.3 |
0.860 |
|
Post TL (°) |
-24.2 ± 15.7 |
-25.3 ± 16.3 |
0.730 |
|
Last TL (°) |
-15.3 ± 22 |
-22.2 ± 17 |
0.100 |
|
Pre LL (°) |
5.8 ± 15.6 |
10.1 ± 15.9 |
0.206 |
|
Pre PI-LL |
61.6 ± 18.1 |
66.2 ± 19.3 |
0.255 |
|
Post LL (°) |
-70.8 ± 8.7 |
-69 ± 11.5 |
0.395 |
|
LL correction (°) |
-76.7 ± 18.4 |
-79.1 ± 16.9 |
0.521 |
|
Post PI-LL |
-15.1 ± 11.2 |
-13 ± 12.8 |
0.407 |
|
Last LL (°) |
-65.8 ± 25.4 |
-62.8 ± 23.5 |
0.577 |
|
Pre LS (°) |
-6.3 ± 15.7 |
0 ± 16.1 |
0.065 |
|
Post LS (°) |
-26.9 ± 9.4 |
-26 ± 8.8 |
0.647 |
|
LS correction (°) |
-20.6 ± 17 |
-26.1 ± 16.7 |
0.134 |
|
Last LS (°) |
-27.4 ± 9.5 |
-25.5 ± 11.6 |
0.412 |
|
PI (°) |
55.8 ± 10.7 |
56.1 ± 10.4 |
0.902 |
|
Pre SS (°) |
24.5 ± 10.5 |
19.8 ± 12.3 |
0.054 |
|
Post SS (°) |
46.1 ± 7.1 |
44.2 ± 8.4 |
0.262 |
|
Last SS (°) |
45.3 ± 8.6 |
42.4 ± 10.3 |
0.152 |
|
Pre PT (°) |
31.3 ± 13.9 |
36.3 ± 11.6 |
0.071 |
|
Post PT (°) |
11.3 ± 6.5 |
14.3 ± 8.5 |
0.073 |
|
Last PT (°) |
13.2 ± 9.3 |
17.4 ± 12.4 |
0.076 |
|
Coronal parameters |
|||
|
Pre coronal Cobb angle (°) |
12.9 ± 10.4 |
13.2 ± 11.4 |
0.925 |
|
Post coronal Cobb angle (°) |
1.5 ± 1 |
1.6 ± 0.7 |
0.373 |
|
Last coronal Cobb angle (°) |
1.7 ± 1.1 |
2.1 ± 1.1 |
0.066 |
|
Pre coronal C7PL (mm) |
18.4 ± 14.8 |
12.9 ± 12.3 |
0.066 |
|
Post coronal C7PL (mm) |
12.6 ± 10 |
11.9 ± 10.4 |
0.756 |
|
Last coronal C7PL (mm) |
11.7 ± 9.5 |
14.2 ± 11.3 |
0.257 |
|
Lower extremity OA parameters |
|||
|
Pre structural LLD (cm) |
0.4 ± 0.3 |
0.7 ± 0.5 |
0.001* |
|
Pre functional LLD (cm) |
0.4 ± 0.3 |
0.6 ± 0.4 |
0.002* |
|
Pre pelvic obliquity (°) |
1.2 ± 1.3 |
2.3 ± 1.8 |
0.002* |
|
Right mechanical axis (°) |
-3.3 ± 4.4 |
-4.3 ± 5.6 |
0.351 |
|
Left mechanical axis (°) |
-3.3 ± 4.9 |
-3.6 ± 6.9 |
0.810 |
|
Right knee OA grade |
2.6 ± 1.2 |
2.5 ± 1.4 |
0.687 |
|
Left knee OA grade |
2.5 ± 1.2 |
2.3 ± 1.5 |
0.467 |
|
Right hip OA grade |
2.2 ± 0.9 |
2.5 ± 0.8 |
0.095 |
|
Left hip OA grade |
2.2 ± 0.9 |
2.5 ± 0.7 |
0.067 |
|
Clinical outcomes |
|||
|
Pre ODI |
37.8 ± 1.7 |
37.9 ± 2.8 |
0.858 |
|
Post ODI |
16.4 ± 8.1 |
18.7 ± 5.9 |
0.126 |
|
Last ODI |
7.5 ± 5.1 |
9.3 ± 3.9 |
0.058 |
|
Pre LBP VAS |
8.2 ± 0.9 |
8.3 ± 1 |
0.325 |
|
Post LBP VAS |
2.4 ± 1.5 |
4 ± 2 |
<0.05* |
|
Last LBP VAS |
1.6 ± 1.4 |
1.8 ± 1.3 |
0.357 |
|
Pre Leg VAS |
8 ± 0.7 |
7.9 ± 1.1 |
0.388 |
|
Post Leg VAS |
1.9 ± 0.7 |
1.8 ± 0.8 |
0.762 |
|
Last Leg VAS |
0.5 ± 0.6 |
1.1 ± 1 |
<0.05* |
|
* Statistically significant (P-value < 0.05) RF : rod fracture; BMD: bone mineral density; BMI: body mass index; Pre: preoperative; Post: postoperative; Last: last follow-up; SVA: sagittal vertical axis; TK: thoracic kyphosis; TL: thoracolumbar junctional angle; LL: lumbar lordosis; PI: pelvic incidence; LS: lumbosacral junctional angle; SS: sacral slope; PT: pelvic tilt; C7PL: C7 plumb line; OA: osteoarthritis; LLD: leg length discrepancy; ODI: Oswestry disability index; VAS: visual analog scale |
|||
Table 1: Continuous variable comparison of risk factors between RF group and Non-RF group.
3.6 Joint pathologies as RF risk factors (Tables 2-4)
Preoperatively, the difference in degenerative lumbar scoliosis and OA between the two hip joints did not differ between the groups. However, the difference in OA between the knee joints was greater, and knee angular deformity was more frequently observed, in the RF group than in the non-RF group (P < 0.05). Additionally, clinically significant structural and functional LLDs were relatively more common in the RF group than in the non-RF group (P < 0.05). On correlation analysis, PO was not correlated with the coronal Cobb angle and coronal C7PL, but was correlated with structural and functional LLDs (P < 0.05). Structural and functional LLDs were also correlated with the mechanical axis difference between the knee joints. Similarly, these joint pathologies were significantly related to RF on multilinear regression analysis (r = 0.546). Specifically, the knee OA difference (unstandardized ß = 1.047), structural LLD (unstandardized ß = 1.771), and knee angular deformity (unstandardized ß = 1.390) were significantly associated with RF (P < 0.05), with larger values indicating a greater risk of RF.
|
Variables |
RF group (n=41) |
Non-RF group (n=48) |
Odds ratio (95 % Cl) |
P-value |
|
Preoperative DLS (Yes/No) |
18/23 |
24/24 |
0.78 (0.34:1.81) |
0.671 |
|
Knee OA difference (Yes/No) |
21/20 |
11/37 |
3.53 (1.42:8.77) |
0.008* |
|
Hip OA difference (Yes/No) |
15/26 |
10/38 |
2.19 (0.85:5.63) |
0.155 |
|
Structural LLD (Yes/No) |
15/26 |
3/45 |
8.65 (2.29:32.73) |
0.000* |
|
Functional LLD (Yes/No) |
9/32 |
2/46 |
6.47 (1.31:31.95) |
0.020* |
|
Knee angular deformity (Yes/No) |
15/26 |
6/42 |
4.04 (1.39:11.72) |
0.012* |
|
* Statistically significant (P-value < 0.05) RF: rod fracture; CI: confidence interval; DLS: degenerative lumbar scoliosis; OA: osteoarthritis; LLD: leg length discrepancy |
||||
Table 2: Categorical variables comparison of risk factors between RF group and Non-RF group.
|
CorCobb |
CorC7PL |
PO |
SLLD |
FLLD |
|
|
Post PO |
- |
- |
0.674 ** |
0.411 ** |
0.574 ** |
|
PO |
- |
- |
- |
0.429 ** |
0.709 ** |
|
MD |
0.306 ** |
0.404 ** |
|||
|
** Significant correlations was established at the 0.01 level CorCobb: coronal cobb angle; CorC7PL: coronal C7 plumb line; Post: postoperative; PO: pelvic obliquity; FLLD: functional leg length discrepancy; SLLD: structural leg length discrepancy; Post: postoperative; MD: mechanical axis difference |
|||||
Table 3: Correlations between radiographic parameters.
|
B |
SE |
Wald χ2 |
P-value |
Odds ratio |
95% CI |
|
|
Knee OA difference |
1.047 |
0.521 |
4.041 |
0.044* |
2.848 |
1.027-7.9 |
|
Structural LLD |
1.771 |
0.718 |
6.088 |
0.014* |
5.874 |
1.439-23.975 |
|
Knee angular deformity |
1.390 |
0.598 |
5.396 |
0.020* |
4.014 |
1.243-12.969 |
|
Constant |
-1.163 |
0.347 |
11.229 |
0.001 |
0.313 |
|
|
* Statistically significant (P-value < 0.05) RF: rod fracture; SE: standard error; CI: confidence interval; OA: osteoarthritis; LLD: leg length discrepancy |
||||||
Table 4: Multivariate logistic regression analysis of the influencing factors of RF
3.7 Clinical outcomes (Table 1)
For both groups, ODI and VAS scores for back pain and radiating pain were improved postoperatively and at the last follow-up compared to preoperative values. However, the postoperative VAS score for low back pain and leg pain at the last follow-up were significantly greater in the RF group than in the non-RF group.
4. Discussion
In the current study, long-segment fixation from T10 to S1 with PSO was performed in 89 patients with ASD for deformity correction, and RF was observed in 41 patients. The target parameters of deformity correction (e.g. postoperative SVA correction, PI-LL, and PT) and other radiographic spinopelvic parameters were not significantly different between RF and non-RF groups with desirable sagittal and coronal radiological balance until the last follow-up. Additionally, patient factors, including sex, BMD, and BMI, were not significantly different between the two groups. We consider that the use of consistent surgical techniques (including PSO) and instruments in elderly patients with a single etiology of LDK led to these outcomes.
4.1 PSO and RF
In a study of 178 patients with LDK (mean age, 70.8 years), PSO and severe preoperative sagittal spinopelvic malalignment status, including preoperative PI-LL mismatch, were revealed as important risk factors for RF after deformity correction [5]. However, PSO is a major deformity correction method for patients with LDK who have relatively large PI or a severe, rigid, and fixed deformity [23]. Therefore, various spinal instruments (e.g. thicker and stronger rods, iliac screws, and accessory rods) have been developed to reduce RF after deformity correction including PSO [5]. However, additional methods that could fundamentally reduce the incidence of RF preoperatively in patients with ASD undergoing PSO are needed. Interestingly, we found that pre-existing lower-extremity joint pathologies were revealed as potential risk factors for RF after deformity correction in ASD.
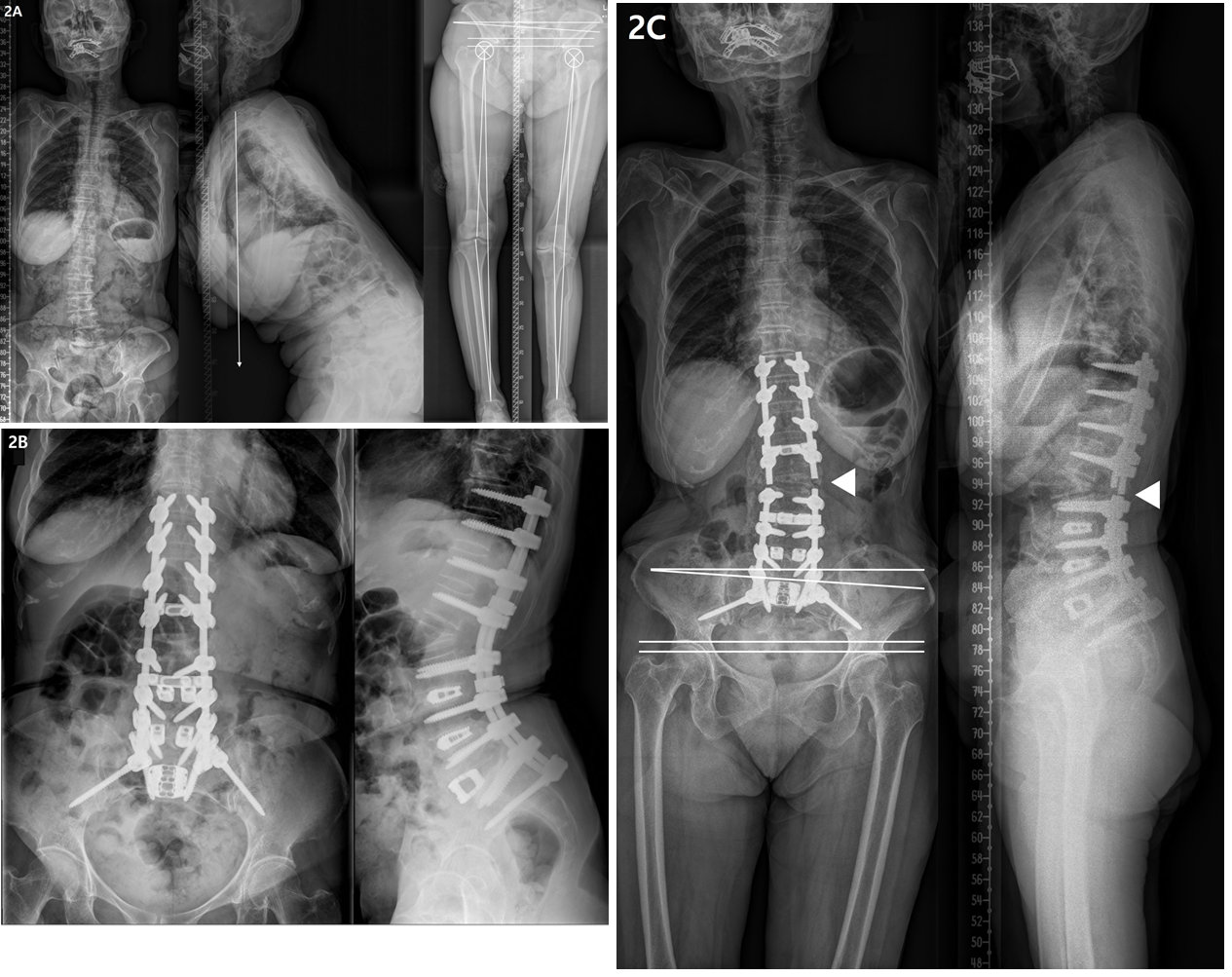
4.2 Lower-extremity OA and LLD in patients with ASD (Figure 2)
Most of our patients with ASD aged >65 years showed spinal degenerative changes and Kellgren-Lawrence OA grade 2 or higher in the knee and hip joints. Lower-extremity OA leads to articular cartilage degeneration, bone hypertrophy at the joint margins, and synovial membrane thickening [24]. In the advanced stage, articulating bone becomes severely deformed [25]. Therefore, OA of the lower-extremity joints may reduce the length of the affected leg and cause angular deformity, which may result in LLD [26-28].
LLD is associated with postural and functional changes not only in the lower extremities, but also in the pelvis and spine [29]. In most studies, LLD <20 mm has been found to be clinically insignificant [30], but although postural adjustments can compensate for the asymmetry, repetitive mechanical loading over time may lead to problems in mild LLD cases [31]. In a study of 225 chiropractic patients, patients with mild LLD (5-15 mm) had a significantly increased prevalence of degenerative joint diseases in L5-S1 spinal motion segments and L4-5 segments [26]. In particular, the prevalence of degenerative changes in elderly individuals aged ≥50 years were significantly increased in those with LLD than in those without LLD. Another study of 19 healthy subjects with artificially induced LLD (by wearing sandals with 1.45-cm height) showed that compensatory changes occur in mild LLD to equalize the functional length of the lower limbs during gait [32]. Thus, mild LLD should not be overlooked in clinical settings. In the present study, although both groups showed mild functional and structural LLD, it was more severe in the RF group than in the non-RF group. Additionally, logistic regression analysis revealed structural LLD as an important risk factor for RF. In a study of 100 patients (mean age, 40 years) with chronic low back pain, LLD (mean, 5 mm) was correlated with PO [33]. In a study of LLD using 1–5 cm foot lifts, the major compensation was in PO for low LLD, up to 2.2 cm. [34] Consistent with these previous studies, we observed a correlation between PO and structural and functional LLDs. However, PO was not correlated with the coronal Cobb angle and coronal C7PL, suggesting that the PO due to LLD could not be corrected, regardless of ideal spinal deformity correction. Therefore, LLD must be corrected first in order to correct PO, and this would reduce RF during deformity correction in ASD.
Figure 2: Radiographs showing a 75-year-old female with degenerative lumbar kyphosis who underwent T10-S1 posterior instrumentation, PSO on L2, PLIF on L3-5, ALIF on L5S1, and sacropelvic fixation; (A) Preoperative whole spine lateral radiograph (SVA, +274 mm; PI, 63°; TK, -18°; LL, 36°; PT, 50°; SS, 13°) and orthogram (Rt. and Lt. hip OA, grade 3; Rt. knee OA, grade 2; Lt. knee OA, grade 4; SLLD, 1.6cm; FLLD, 1.4cm; PO, 3.9°; Rt. and Lt. MA difference, 14°). (B) Postoperatively, spinal deformity correction was successfully performed with ideal lumbar lordosis and optimal sagittal balance (SVA, -42 mm; TK, 18°; LL, -68°; PT, 23°; SS, 40°; PI-LL, -5). (C) RF occurred 15 months after deformity correction, with the joint pathologies persisted.
4.3 Knee joint OA and RF
In the present study, OA showed a similar pattern in both hip joints. However, the difference in OA between right and left knee joints was relatively greater, and knee angular deformity was also more common, in the RF group than in the non-RF group. Furthermore, these parameters were important risk factors for RF. In gait analysis studies of patients with hip fractures and knee joint pathologies [35-38], asymmetric gait persisted for >2 years after surgical procedures involving the gluteal muscle, manifesting as antalgic gait in patients with gonarthrosis. Therefore, continuous tensile force from lateral bending at the mid-stance phase and continuous compressive force on the contralateral side from less shock absorption in the arthritic knee joint, as well as axial rotational force from the trunk during the swing phase, seem to be coronal mechanisms for RF during asymmetric gait. Knee OA is the most common type of OA [39]. Although the mechanism is not well established, OA is usually more symptomatic and severe on one side of the knee than on the other side [39]. Therefore, in surgical treatment for patients with ASD, the evaluation of knee OA must be prioritized. In cases of severe OA, differences between knees, and PO with LLD, knee OA should be treated prior to performing spinal deformity correction.
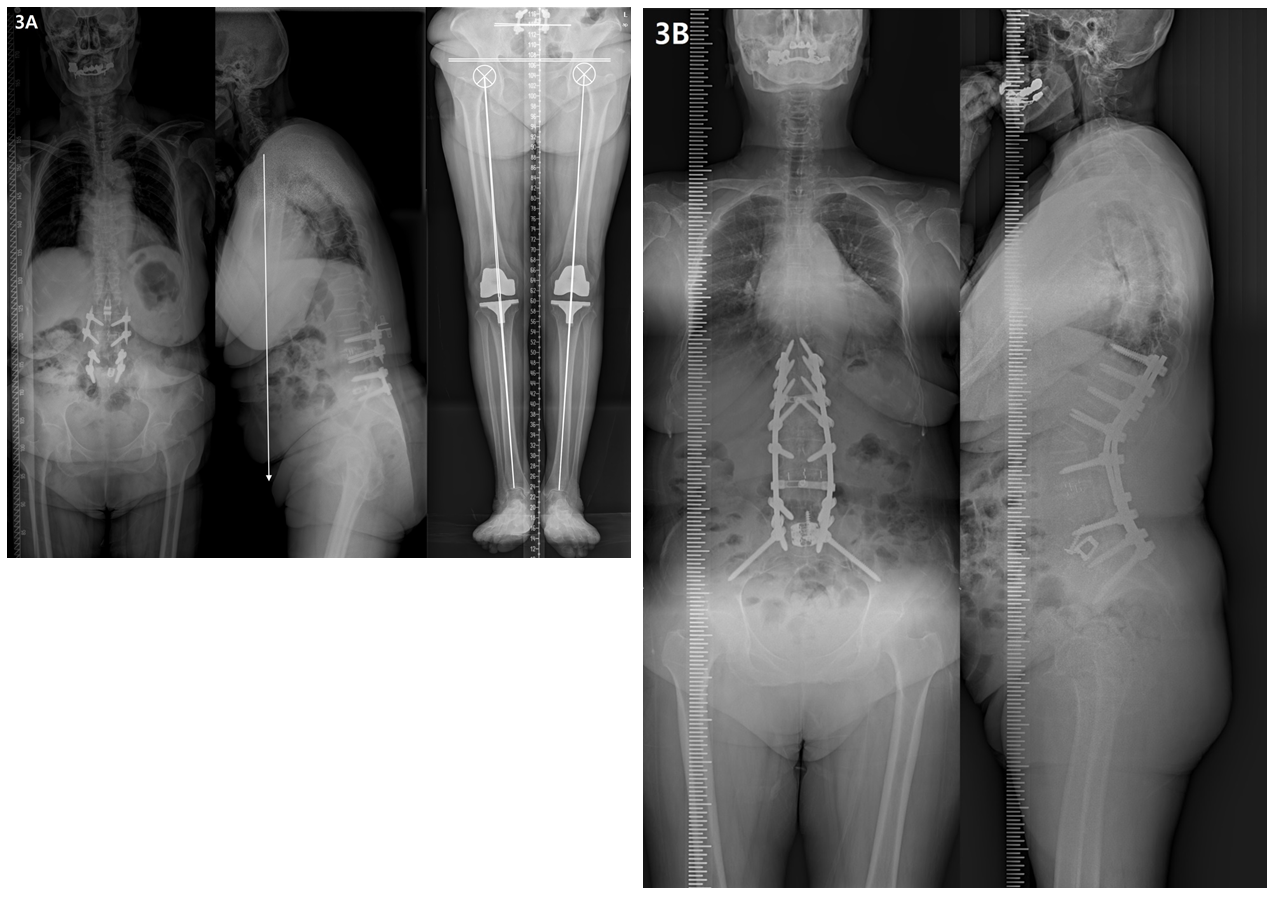
4.4 Elderly patients and the future of treatment (Figure 3)
The prevalence of OA tends to increase with age, and 30%–50% of adults aged >65 years develop OA [24]. Elderly patients commonly develop OA in several areas, and the effects and interactions of OA in different areas have significant effects on disabilities in daily life [40]. Recent developments in medical technology have led to various osteotomy techniques, surgical methods, and instruments for spinal deformity correction. Additionally, treatment strategies for OA in other areas (e.g. arthroplasty of the hip and knee joints) are showing rapid progress. Therefore, with these developments, the treatment for elderly patients must consider the overall body, including multiple joints, and focus on improving the quality of life of patients through appropriate surgical treatment of the spine and every joint to maintain an active senior lifestyle.
Figure 3: Radiographs showing a 74-year-old female with degenerative lumbar kyphosis who underwent T10-S1 posterior instrumentation, PSO on L2, ALIF on L5-S1, and sacropelvic fixation. (A) Preoperative whole spine lateral radiograph (SVA, +192 mm; PI, 41°; TK, 17°; LL, 6°; PT, 25°; SS, 16°) and orthogram (Rt. and Lt. hip OA, grade 2; Rt. and Lt. TKR state; SLLD, 0.3cm; FLLD, 0.2cm; PO, 1.1°; Rt. and Lt. MA difference, 0.7°). (B) Postoperative 2-year radiograph showing a well-maintained sagittal balance (SVA, +7 mm; TK, 50°; LL, -63°; PT, 4°; SS, 37°) without RF.
4.5 Limitations
This study has some limitations, including its retrospective design. There are several reported risk factors for pseudarthrosis including RF after PSO in ASD [41-44]. Thus, RF may be caused not only by a few factors, but also by the interaction of multiple factors. Accordingly, if RF occurs after deformity correction for ASD, a thorough causal investigation is required, and the risk factors demonstrated in the present study may not have been the cause of RF. However, as the purpose of this study was to seek strategies to fundamentally reduce RF before surgery, its findings, that preferential treatment of lower-extremity joint pathologies before surgery may reduce the incidence of RF, are meaningful.
5. Conclusion
We found that ASD in elderly patients is often accompanied by degenerative changes in the lower extremities, and concluded that even if spine deformity correction is successfully performed, complications, including RF, can occur if joint pathologies are not resolved. Therefore, when performing deformity correction in patients with ASD, it is important to consider perioperative treatments for the lower extremities, including the knee joint, as well as the restoration of the spine and pelvis.
6. Declaration
Acknowledgments
No additional person beyond the authors contributed to this study.
* We certify that no portion of this manuscript has been previously presented.
* We would like to thank Editage (www.editage.co.kr) for English language editing.
Authors contribution
Conception and design: KYL, JHL, Data collection: KYL, SKI, Data analysis and interpretation: KYL, SKI, Manuscript drafting: KYL, Manuscript revision: JHL, Approval of the final manuscript: All the authors.
Funding
There were no financial and material support received for this work from any of the following organizations: National Institutes of Health (NIH); Wellcome Trust; Howard Hughes Medical Institute (HHMI); and others
Availability of Data and Materials
The datasets used for this study are available from the corresponding author on request.
Ethical Approval
This study was approved by the ethics committee of our institution (KMC IRB 2021-06-030).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine 30 (2005): 1082-1085.
- Lee J-H, Kim K-T, Lee S-H, et al. Overcorrection of lumbar lordosis for adult spinal deformity with sagittal imbalance: comparison of radiographic outcomes between overcorrection and undercorrection. Eur Spine J 25 (2016): 2668-2675.
- Bridwell KH, Lewis SJ, Edwards C, et al. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine 28 (2003): 2093-2101.
- Hyun SJ, Rhim SC. Clinical outcomes and complications after pedicle subtraction osteotomy for fixed sagittal imbalance patients: a long-term follow-up data. J Korean Neurosurg Soc 47 (2010): 95-101.
- Lee KY, Lee JH, Kang KC, et al. Strategies for prevention of rod fracture in adult spinal deformity: cobalt chrome rod, accessory rod technique, and lateral lumbar interbody fusion. J Neurosurg Spine (2021): 1-10.
- Lee KY, Lee JH, Kang KC, et al. Strategy for obtaining solid fusion at L5-S1 in adult spinal deformity: risk factor analysis for nonunion at L5-S1. J Neurosurg Spine (2020): 1-9.
- Lafage R, Schwab F, Challier V, et al. Defining spino-pelvic alignment thresholds: should operative goals in adult spinal deformity surgery account for age? Spine 41 (2016): 62-68.
- Miyagi M, Fukushima K, Inoue G, et al. Hip-spine syndrome: cross-sectional-study of spinal alignment in patients with coxalgia. Hip Int 29 (2019): 21-25.
- Murata Y, Takahashi K, Yamagata M, et al. The knee-spine syndrome. Association between lumbar lordosis and extension of the knee. J Bone Joint Surg Br 85 (2003): 95-99.
- Takemitsu Y, Harada Y, Iwahara T, et al. Lumbar degenerative kyphosis. Clinical, radiological and epidemiological studies. Spine 13 (1988): 1317-1326.
- Lee C-S, Lee C-K, Kim Y-T, et al. Dynamic sagittal imbalance of the spine in degenerative flat back: significance of pelvic tilt in surgical treatment. Spine 26 (2001): 2029-2035.
- Lee C-S, Kim Y-T, Kim E. Clinical study of lumbar degenerative kyphosis. J Korean Soc Spine Surg 4 (1997): 27-35.
- Chang KW, Cheng CW, Chen HC, et al. Closing-opening wedge osteotomy for the treatment of sagittal imbalance. Spine 33 (2008): 1470-1477.
- Horton WC, Brown CW, Bridwell KH, et al. Is there an optimal patient stance for obtaining a lateral 36” radiograph?: a critical comparison of three techniques. Spine 30 (2005): 427-433.
- Langella F, Villafane JH, Damilano M, et al. Predictive accuracy of Surgimap surgical planning for sagittal imbalance: a cohort study. Spine 42 (2017): 1297-1304.
- Roussouly P, Pinheiro-Franco JL. Sagittal parameters of the spine: biomechanical approach. Eur Spine J 20 (2011): 578-585.
- Lowe T, Berven SH, Schwab FJ, et al. The SRS classification for adult spinal deformity: building on the King/Moe and Lenke classification systems. Spine 31 (2006): 119-125.
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16 (1957): 494-502.
- Ploumis A, Trivedi V, Shin JH, et al. Progression of idiopathic thoracic or thoracolumbar scoliosis and pelvic obliquity in adolescent patients with and without limb length discrepancy. Scoliosis Spinal Disord 13 (2018): 18.
- Sekiya T, Aota Y, Yamada K, et al. Evaluation of functional and structural leg length discrepancy in patients with adolescent idiopathic scoliosis using the EOS imaging system: a prospective comparative study. Scoliosis Spinal Disord 13 (2018): 7.
- Piyakunmala K, Sangkomkamhang T. Measurement of patient's perception on limb-length discrepancy compared with weight-bearing orthoroentgenography in total hip arthroplasty: a prospective study. J Arthroplasty 33 (2018): 2301-2305.
- Subburaj K, Ravi B, Agarwal M. Computer-aided methods for assessing lower limb deformities in orthopaedic surgery planning. Comput Med Imaging Graph 34 (2010): 277-288.
- Kim KT, Park KJ, Lee JH. Osteotomy of the spine to correct the spinal deformity. Asian Spine J 3 (2009): 113-123.
- Loeser RF. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med 26 (2010): 371-386.
- Solomon L. Patterns of osteoarthritis of the hip. J Bone Joint Surg Br 58 (1976): 176-183.
- Murray KJ, Molyneux T, Le Grande MR, et al. Association of mild leg length discrepancy and degenerative changes in the hip joint and lumbar spine. J Manipulative Physiol Ther 40 (2017): 320-329.
- Friend L, Widmann RF. Advances in management of limb length discrepancy and lower limb deformity. Curr Opin Pediatr 20 (2008): 46-51.
- Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum 58 (2008): 1716-1726.
- Murray KJ, Azari MF. Leg length discrepancy and osteoarthritis in the knee, hip and lumbar spine. J Can Chiropr Assoc 59 (2015): 226-237.
- Gross RH. Leg length discrepancy: how much is too much? Orthopedics 1 (1978): 307-310.
- Subotnick SI. Limb length discrepancies of the lower extremity (the short leg syndrome). J Orthop Sports Phys Ther 3 (1981): 11-16.
- Resende RA, Kirkwood RN, Deluzio KJ, et al. Biomechanical strategies implemented to compensate for mild leg length discrepancy during gait. Gait Posture 46 (2016): 147-153.
- Hoikka V, Ylikoski M, Tallroth K. Leg-length inequality has poor correlation with lumbar scoliosis. A radiological study of 100 patients with chronic low-back pain. Arch Orthop Trauma Surg 108 (1989): 173-175.
- Walsh M, Connolly P, Jenkinson A, et al. Leg length discrepancy--an experimental study of compensatory changes in three dimensions using gait analysis. Gait Posture 12 (2000): 156-161.
- Jarnlo GB, Thorngren KG. Standing balance in hip fracture patients. 20 middle-aged patients compared with 20 healthy subjects. Acta Orthop Scand 62 (1991): 427-434.
- Creaby MW, Bennell KL, Hunt MA. Gait differs between unilateral and bilateral knee osteoarthritis. Arch Phys Med Rehabil 93 (2012): 822-827.
- Mills K, Hettinga BA, Pohl MB, et al. Between-limb kinematic asymmetry during gait in unilateral and bilateral mild to moderate knee osteoarthritis. Arch Phys Med Rehabil 94 (2013): 2241-2247.
- Kneiss JA, Hilton TN, Tome J, et al. Weight-bearing asymmetry in individuals post-hip fracture during the sit to stand task. Clin Biomech 30 (2015): 14-21.
- Noll DR. Leg length discrepancy and osteoarthritic knee pain in the elderly: an observational study. J Am Osteopath Assoc 113 (2013): 670-678.
- Keenan AM, Tennant A, Fear J, et al. Impact of multiple joint problems on daily living tasks in people in the community over age fifty-five. Arthritis Rheum 55 (2006): 757-764.
- Smith JS, Shaffrey E, Klineberg E, et al. Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J Neurosurg Spine 21 (2014): 994-1003.
- Kim YJ, Bridwell KH, Lenke LG, et al. Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine 30 (2005): 468-474.
- Berjano P, Bassani R, Casero G, et al. Failures and revisions in surgery for sagittal imbalance: analysis of factors influencing failure. Eur Spine J 22 (2013): 853-858.
- Dickson DD, Lenke LG, Bridwell KH, et al. Risk factors for and assessment of symptomatic pseudarthrosis after lumbar pedicle subtraction osteotomy in adult spinal deformity. Spine 39 (2014): 1190-1195.