Implications of Obstructive Sleep-related Breathing Disorder in Dentistry: Focus on Snoring and Obstructive Sleep Apnea
Article Information
Yeon-Hee Lee*
1Department of Orofacial Pain and Oral Medicine, Kyung Hee University Dental Hospital, Kyung Hee Medical center, Kyung Hee University, Seoul, Korea
*Corresponding Author: Yeon-Hee Lee, Department of Orofacial Pain and Oral Medicine, Kyung Hee University Dental Hospital, Kyung Hee Medical center, Kyung Hee University, Seoul, Korea
Received: 29 September 2022; Accepted 03 October 2022; Published: 05 October 2022
Citation:
Yeon-Hee Lee. Investigation of Snoring and Obstructive Sleep Apnea Using Portable Polysomnography in Patients with Temporomandibular Disorder. Dental Research and Oral Health 5 (2022): 074-082.
View / Download Pdf Share at FacebookAbstract
Obstructive sleep-related breathing disorder (SRBD) is an umbrella term that encompasses various types of upper airway dysfunctions during sleep characterized by increased respiratory effort secondary to snoring and/or increased upper airway resistance and pharyngeal collapse. Obstructive sleep apnea (OSA) is a representative SRBD that involves a significant decrease in or cessation of airflow despite the presence of respiratory effort. While snoring is considered a normal condition, it can cause serious noise disturbance to sleep partners and is considered a predictor of OSA. Snoring and OSA are highly correlated with obesity. SRBDs can lead to cardiovascular disease, hypertension, decreased quality of life, decreased work efficiency, daytime sleepiness, decreased neurocognitive activity, and psychological impairments. In dentistry, research on sleep problems has focused on temporomandibular disorder (TMD)/orofacial pain. The relationship between OSA and TMD/orofacial pain has been reported, but it is not clear whether it is a simple correlation or a causal relationship. Therefore, we aimed to review the causes of SRBDs including snoring and OSA and to review and infer the relationship between these SRBDs and TMD/orofacial pain. The effects of snoring and OSA extend beyond sleep disturbances and are worthy of future research, especially with regard to TMD.
Keywords
Sleep-related breathing disorder, Snoring, Obstructive sleep apnea, Temporomandibular disorder, Orofacial pain, Dentistry
Sleep-related breathing disorder articles; Snoring articles; Obstructive sleep apnea articles; Temporomandibular disorder articles; Orofacial pain articles; Dentistry articles
Sleep-related breathing disorder articles Sleep-related breathing disorder Research articles Sleep-related breathing disorder review articles Sleep-related breathing disorder PubMed articles Sleep-related breathing disorder PubMed Central articles Sleep-related breathing disorder 2023 articles Sleep-related breathing disorder 2024 articles Sleep-related breathing disorder Scopus articles Sleep-related breathing disorder impact factor journals Sleep-related breathing disorder Scopus journals Sleep-related breathing disorder PubMed journals Sleep-related breathing disorder medical journals Sleep-related breathing disorder free journals Sleep-related breathing disorder best journals Sleep-related breathing disorder top journals Sleep-related breathing disorder free medical journals Sleep-related breathing disorder famous journals Sleep-related breathing disorder Google Scholar indexed journals Snoring articles Snoring Research articles Snoring review articles Snoring PubMed articles Snoring PubMed Central articles Snoring 2023 articles Snoring 2024 articles Snoring Scopus articles Snoring impact factor journals Snoring Scopus journals Snoring PubMed journals Snoring medical journals Snoring free journals Snoring best journals Snoring top journals Snoring free medical journals Snoring famous journals Snoring Google Scholar indexed journals Obstructive sleep apnea articles Obstructive sleep apnea Research articles Obstructive sleep apnea review articles Obstructive sleep apnea PubMed articles Obstructive sleep apnea PubMed Central articles Obstructive sleep apnea 2023 articles Obstructive sleep apnea 2024 articles Obstructive sleep apnea Scopus articles Obstructive sleep apnea impact factor journals Obstructive sleep apnea Scopus journals Obstructive sleep apnea PubMed journals Obstructive sleep apnea medical journals Obstructive sleep apnea free journals Obstructive sleep apnea best journals Obstructive sleep apnea top journals Obstructive sleep apnea free medical journals Obstructive sleep apnea famous journals Obstructive sleep apnea Google Scholar indexed journals Temporomandibular disorder, articles Temporomandibular disorder, Research articles Temporomandibular disorder, review articles Temporomandibular disorder, PubMed articles Temporomandibular disorder, PubMed Central articles Temporomandibular disorder, 2023 articles Temporomandibular disorder, 2024 articles Temporomandibular disorder, Scopus articles Temporomandibular disorder, impact factor journals Temporomandibular disorder, Scopus journals Temporomandibular disorder, PubMed journals Temporomandibular disorder, medical journals Temporomandibular disorder, free journals Temporomandibular disorder, best journals Temporomandibular disorder, top journals Temporomandibular disorder, free medical journals Temporomandibular disorder, famous journals Temporomandibular disorder, Google Scholar indexed journals Orofacial pain articles Orofacial pain Research articles Orofacial pain review articles Orofacial pain PubMed articles Orofacial pain PubMed Central articles Orofacial pain 2023 articles Orofacial pain 2024 articles Orofacial pain Scopus articles Orofacial pain impact factor journals Orofacial pain Scopus journals Orofacial pain PubMed journals Orofacial pain medical journals Orofacial pain free journals Orofacial pain best journals Orofacial pain top journals Orofacial pain free medical journals Orofacial pain famous journals Orofacial pain Google Scholar indexed journals Dentistry articles Dentistry Research articles Dentistry review articles Dentistry PubMed articles Dentistry PubMed Central articles Dentistry 2023 articles Dentistry 2024 articles Dentistry Scopus articles Dentistry impact factor journals Dentistry Scopus journals Dentistry PubMed journals Dentistry medical journals Dentistry free journals Dentistry best journals Dentistry top journals Dentistry free medical journals Dentistry famous journals Dentistry Google Scholar indexed journals temporomandibular joint articles temporomandibular joint Research articles temporomandibular joint review articles temporomandibular joint PubMed articles temporomandibular joint PubMed Central articles temporomandibular joint 2023 articles temporomandibular joint 2024 articles temporomandibular joint Scopus articles temporomandibular joint impact factor journals temporomandibular joint Scopus journals temporomandibular joint PubMed journals temporomandibular joint medical journals temporomandibular joint free journals temporomandibular joint best journals temporomandibular joint top journals temporomandibular joint free medical journals temporomandibular joint famous journals temporomandibular joint Google Scholar indexed journals OSA syndrome articles OSA syndrome Research articles OSA syndrome review articles OSA syndrome PubMed articles OSA syndrome PubMed Central articles OSA syndrome 2023 articles OSA syndrome 2024 articles OSA syndrome Scopus articles OSA syndrome impact factor journals OSA syndrome Scopus journals OSA syndrome PubMed journals OSA syndrome medical journals OSA syndrome free journals OSA syndrome best journals OSA syndrome top journals OSA syndrome free medical journals OSA syndrome famous journals OSA syndrome Google Scholar indexed journals sleep segmentation articles sleep segmentation Research articles sleep segmentation review articles sleep segmentation PubMed articles sleep segmentation PubMed Central articles sleep segmentation 2023 articles sleep segmentation 2024 articles sleep segmentation Scopus articles sleep segmentation impact factor journals sleep segmentation Scopus journals sleep segmentation PubMed journals sleep segmentation medical journals sleep segmentation free journals sleep segmentation best journals sleep segmentation top journals sleep segmentation free medical journals sleep segmentation famous journals sleep segmentation Google Scholar indexed journals
Article Details
Introduction
Sleep and pain have a bidirectional relationship. In other words, disturbances in sleep exacerbate pain or lead to persistent pain, and the presence of pain interferes with sleep [1]. During sleep, upper airway patency decreases, and upper airway resistance on ventilation increases. Obstructive sleep-related breathing disorder (SRBD) is a broad term encompassing various upper airway dysfunctions during sleep characterized by increased respiratory effort secondary to snoring and/or greater upper airway resistance and pharyngeal collapse [2]. SRBD is a group of disorders including upper airway resistance syndrome, central sleep apnea, and obstructive sleep apnea (OSA). SRBD can lead to cardiovascular disease, hypertension, decreased quality of life, decreased work efficiency, daytime sleepiness, deteriorated neurocognitive activity, increased systemic inflammation, and psychological impairment [3]. OSA is a representative SRBD with crucial implications beyond interference with sleep partners. OSA is a sleep disorder in which airflow is significantly reduced or stopped in the presence of respiratory effort [4,5]. Patients with OSA experience collapse of the upper airway during sleep, which is a hallmark sign. OSA is an independent risk factor for cardiovascular, neurological, and psychological morbidities. Although snoring is considered a physiologically normal condition with noisy sounds, such as a growling or gasping sound, it can cause serious noise disturbance for sleep partners and can be a predictor of OSA [6,7]. In general, snoring and OSA are highly correlated with obesity and anatomical narrowing of the upper airway. In patients with OSA, hypoxemia and sleep segmentation during sleep can activate the sympathetic nervous system and increase oxidative stress. Inflammatory responses are assumed to be the main mechanisms responsible for the diseases mentioned above. In dentistry, the relationship between OSA, systemic inflammation, and temporomandibular disorder (TMD)/orofacial pain has been reported [8]. There is still a lack of evidence on whether a simple correlation, causal relationship, or comorbidity exists between OSA and TMD/orofacial pain. Therefore, this narrative review aimed to review the etiology of obstructive SRBD including snoring and OSA, to explain the pathophysiology of obstructive sleep disorder respiration, and to review and infer the relationship between OSA and TMD/orofacial pain. This review highlights that the effect of SRBDs, such as snoring and OSA, expands beyond sleep-related problems and is worth examining in future research on TMD.
Materials and Methods
A narrative review was performed based on a search of the PubMed and Google Scholar databases for articles on the etiology, pathophysiology, and role of obstructive SRBDs, including snoring and OSA, in dentistry. The following keywords were used in the search to find related articles: “sleep-related breathing disorder,” “snoring,” “sleep apnea,” “obstructive sleep apnea,” “obstructive sleep apnea syndrome,” “sleep,” “temporomandibular joint (TMJ),” “orofacial,” “orofacial pain,” and “TMD”. Papers published in English over the last 30 years (between January 1992 and August 2022) were searched. A total of 2618 articles were retrieved from the PubMed and Google Scholar databases over the 30-year period from January 1992 to August 2022. After application of the inclusion and exclusion criteria and analyzing the abstracts and full texts of some articles, 82 articles were finally selected. In this review, data on OSA were also taken from articles on OSA syndrome, SRBDs, and sleep apnea, if relevant. In other words, for the purpose of this review, “OSA” included all types of OSA except for central sleep apnea and Cheyne-Stokes breathing. The author repeatedly reviewed the papers over a 3-week period to verify the content and study design and determine whether the papers were suitable for inclusion in this study.
Results
Diagnosis and pathophysiology of snoring
Snoring disorder can occur in anyone and at any age. Snoring can also be a predictor of chronic problems and severe SRBDs. Snoring refers to the sound produced by vibrations of respiratory structures of the upper airway tract during sleep [9]; snoring is caused by vibrations of the oropharynx structure when air passes through relaxed tissues of the nose and throat. Snoring without daytime sleepiness, fatigue, or OSA is simple snoring [10]. According to the International Classification of Sleep Disorders, 3rd edition (ICSD-3), snoring is classified as an SRBD [11].
Snoring can impair bed partners’ sleep as an acoustic disturbance and a potential source of noise pollution. According to previous reports, the prevalence rate of snoring is between 3.8% and 40.3%, and it increases with age [12]. Snoring increased with age and peaked at ages 50-59 in both men and women. In women, menopause was associated with the occurrence of snoring, and women aged 59 and older experienced a less dramatic decrease in snoring than men [13].
The etiologies of snoring are diverse and complex, including anatomical problems of the mouth (thick soft palate and narrow airways) and nose (septum dislocation and chronic nasal congestion), sinusitis, allergies, poor sleep posture, weight gain, overweight, obesity, alcohol consumption, and hormonal changes [14]. Typical anatomical features of obstructive sleep apnea (OSA) in the orofacial area are: (1) an abnormally enlarged tongue; (2) the long soft palate and/or tonsillar enlargement; (3) an extended uvula; (4) high palatal vault; (5) posteriorly positioned mandible (retrognathia) or small-sized mandible; (6) loss of normal occlusion; (7) adenoid hypertrophy; (8) reduced pharyngeal upper airway space; (9) the increased distance between the mandible and the hyoid bone; and (10) increased neck fat deposition surrounding the upper airway (Figure 2) [15,16]. According to Ohayon et al., regular snoring was significantly associated with age >25 years, male sex, obesity, smoking, daytime sleepiness, sleep fragmentation, and high caffeine intake [12]. Snoring can be detected and/or diagnosed using polysomnography, reports from sleep partners, or snoring recording [17].
Anatomical upper airway obstruction may indicate snoring with OSA [18]. In a Hungarian population survey, 37% of male and 21% of female participants with loud snoring had sleep apnea [19]. Snoring and OSA are risk factors for cardiovascular disease, and noise pollution from snoring in excess of 53 dB can cause adverse cardiovascular events in both snorers and sleep partners [20]. In particular, accumulated nighttime exposure to snoring may contribute to the onset and/or progression of cardiovascular disease [21]. Cardiovascular stress increases sympathetic activation, which can lead to spikes in the heart rate and persistently elevated blood pressure during sleep [22]. Furthermore, when SRBDs disrupt the regulation of inflammation by the sympathetic nervous system and neurotransmitters, systemic inflammatory reactions may become uncontrolled or persistent [23]. This can inevitably worsen pain conditions. Nevertheless, objective investigations of the association between snoring severity and OSA are lacking.
Diagnosis of OSA
OSA is characterized by recurrent episodes with cessation of airflow (apnea) or shallow breathing (hypopnea) during sleep despite the presence of respiratory efforts (Figure 1). When the duration of a ≥90% airflow drop in the sensor signal is ≥10 seconds, it counts as an occurrence of an apnea event. The technical definition of hypopnea is shallow breathing lasting more than 10 seconds in which an airflow is reduced by ≥30% [24]. Consequently, hypoxemia, arousal, sleep segmentation, and sympathetic hyperactivity occur repeatedly during sleep [25]. Respiratory effort related arousal (RERA) can occur in patients with OSA. RERA is scored when the inspiratory nasal pressure is flattened for more than 10 seconds leading to arousal from sleep. Arousals in RERA events occur due to respiratory effort, but does not meet the criteria for apnea or hypopnea [26]. The severity of OSA is determined by three representative objective indices. For SRBD and OSA diagnosis, (1) apnea hypopnea index (AHI) or (2) respiratory disturbance index (RDI) can be used if polysomnography is performed, and (3) respiratory event index (REI) can be used if out-of-center sleep testing was accompanied [27].
|
Each OSA index is derived by the following formula: l AHI = number of apneas+hypopneas/total sleep time l RDI = number of apneas+hypopneas +RERAs/total sleep time l REI = number of apneas+hypopneas/monitoring time |
When microarousals or arousals occur in RERA, respiratory effort in OSA patients can be resolved. OSA is a common disease with a prevalence of 3-5%, with asymptomatic patients accounting for up to 26% of the patient population [28]. In the general population, its prevalence is approximately 4% in men and 2% in women [29]. OSA causes excessive daytime sleepiness, fatigue, drowsiness, poor concentration, cognition, and memory loss in daily life. Various factors are associated with OSA: (1) structural factors, such as adenotonsillar hypertrophy, craniofacial abnormality, and obesity; (2) neuromotor factors, such as cerebral palsy and genetic diseases; and (3) other factors, such as hormonal changes and aging. Obesity is a well-known risk factor for OSA [30]. In patients with OSA, apnea and hypopnea during sleep cause hypoxemia, hypercapnia, and frequent arousal, resulting in poor sleep quality at night [31]. Furthermore, OSA has been reported to affect the incidence of depression, psychological disorders, diabetes, coronary artery disease, stroke, pulmonary hypertension, and cardiovascular mortality [32,33].
OSA, the most common subtype of SRBDs, accounts for 95% of all apnea cases [34] but is poorly diagnosed. OSA is currently diagnosed according to the ICSD-3 diagnostic criteria using polysomnography [11]. The detailed OSA criteria of ICSD-3 define OSA as follows: (1) ≥5 respiratory events per hour on a sleep test and accompanying clinical symptoms or comorbidities such as cardiovascular disease and (2) >15 respiratory events per hour on a sleep test [11]. OSA is generally defined as AHI or REI ≥5 [27]. AHI is the sum of the number of apneas and hypopneas per hour during sleep. According to the American Academy of Sleep Medicine, OSA is categorized as normal (<5 events/h), mild OSA (5-15 events/h), moderate (15-30 events/h), and severe (>30 events/h) [35]. Vulnerability to excessive daytime sleepiness varies with the severity of AHI [36]. Polysomnography is the gold standard for the diagnosis of OSA. Level I polysomnography, performed in the presence of a test facilitator, is the most definitive diagnostic method for OSA [37].
The most common symptom in clinical examination of the patients is snoring, which may suggest OSA [38]. Simple snoring may be due to mild obstruction of the upper airway, which is a possible risk factor for the development and progression of OSA. In addition, sleep apnea, in which breathing repeatedly ceases and then resumes, is observed by sleep partners. About a quarter of the patients with OSA complain of daytime sleepiness and various signs or symptoms such as awakening during sleep due to choking or gasping, nocturia, headache after waking up, poor concentration, sensitivity, depression, insomnia, and impotence [39].
On physical examination of patients with OSA, signs such as an increase in waist or neck circumference accompanying obesity or overweight can be observed [40]. A deviated nasal septum, hypertrophy of the turbinate, and a vertically low position of the hyoid bone are also related to the severity of OSA [41]. In addition, the size of the tonsils or upper airway restriction can be confirmed using the Mallampati score or Friedman stage evaluation method [42,43]. The Berlin Questionnaire and STOP-Bang questionnaire are some simple test tools for screening high-risk patients with OSA [44]. The Epworth Sleepiness Scale is commonly used to evaluate daytime sleepiness in patients with OSA [45]. These questionnaires are used as simple tools for screening high-risk groups rather than for diagnosing OSA.
Pathophysiology of OSA
OSA is a heterogeneous syndrome that results from various predispositions, clinical characteristics, respiratory events, and pathophysiological mechanisms. In OSA, recurrent episodes of apnea and hypopnea result in a decrease in oxyhemoglobin saturation and sleep fragmentation and a decrease in the amount of slow waves and rapid eye movement sleep [46]. Although anatomical collapsibility of the upper airway is a very important etiologic factor in apnea and hypopnea events, it is also a consequence of non-anatomical causes and thus cannot be attributed to a single anatomical cause [47]. Phenotypes of OSA are classified according to the major mechanisms of disease development and degrees of anatomical collapsibility of the upper airways, loop gain, and arousal threshold [48].
Regarding anatomical collapse of the upper airway as a mechanism of OSA, the main aspects include narrowing of the upper airway due to anatomical structural problems of the upper airway, collapsing of the oropharynx, and deteriorating pharyngeal patency due to obesity with fat accumulation in soft tissues and the tongue [49]. In addition, the increase in central adipose tissue due to abdominal obesity can increase the collapsibility of the pharynx by reducing lung volume [50]. Collapse of the upper airway during sleep and its collapsibility can be evaluated using the critical closing pressure (Pcrit) method. However, the range of Pcrit in patients with OSA varies, and 20% of patients show values similar to those of the normal individuals, suggesting that OSA is not just a problem of upper airway collapsibility [51]. Commonly used traditional OSA treatments such as continuous positive airway pressure, mandibular advancement device, upper airway surgery, weight loss, and positional therapy can help resolve the issue of anatomical collapsibility [52,53].
However, recent studies have suggested that non-anatomical mechanisms may play an important role in the pathophysiology of OSA. Gray et al. claimed that at least one non-anatomical mechanism contributes to the development of OSA in approximately 70% of patients with OSA [54]. The key factor to be considered in the occurrence of OSA due to non-anatomical mechanisms is the function of muscles of the pharynx and the neuromodulatory action on these muscles [55]. Muscles of the pharynx are important in the development and progression of OSA as they play a key role in keeping the upper airway open.
Another non-anatomical mechanism of OSA is related to lowered arousal threshold. When the increase in negative pressure in the thoracic cavity reaches a certain threshold, cortical awakening occurs during a sleep apnea event [56]. Arousal threshold refers to the degree of breathing effort during arousal. OSA elicits cortical arousal during sleep [57]. Conversely, a lowered arousal threshold is commonly related to OSA occurrence or frequent brief awakenings [58]. Sleep and arousal events are repeated periodically during sleep in patients with OSA, and these repetitions make breathing unstable and interfere with deep sleep, exacerbating OSA [59].
Finally, the non-anatomical mechanism is related to loop gain, which is a control mechanism of human respiration. Loop gain has been used to quantify the internal amplification of a system [60]. In other words, the dimensionless value of the propensity of a system to be controlled by a feedback loop to develop unstable behavior is called the loop gain. The concept of loop gain can be applied to the respiratory system. In the respiratory system of patients with high loop gain, quantified as the ratio of ventilatory response to total respiratory disturbance, ventilatory control is unstable, resulting in an excessive ventilatory response to small changes in CO2 [61,62]. Ventilatory overreaction can lead to hypocapnia, which can worsen sleep apnea by reducing the respiratory drive [63].
Relationship between SRBD and TMD/orofacial pain
TMD is an umbrella term characterized by clinical pain and dysfunction involving the TMJ, masticatory muscles, and their adjacent related structures [64]. TMD is very common in the general population, with a reported prevalence of up to 15% in adults [65]. Possible risk factors for TMD include parafunctional oral habits, macrotrauma, microtrauma, psychological problems, other bodily pain conditions, and sleep problems [64]. The etiopathophysiology of TMD is complex, and TMD is difficult to treat/manage and prone to becoming chronic. Approximately 50% of people with self-reported low sleep quality have comorbid chronic pain [66]. According to the diagnostic criteria for TMD, disc displacement, joint pain, myofascial pain, degenerative and inflammatory joint disease, and headaches attributed to TMD are the major and common subtypes of TMD [67]. Deterioration of sleep quality has been reported in 90% of patients with TMD [68]. At high co-occurrence rates, a close, bidirectional relationship between TMD pain or orofacial pain and sleep disorders can be inferred (Figure 3). In addition, orofacial pain, including TMD pain, and sleep deterioration may have significantly overlapping etiopathophysiologies or underlying mechanisms [68,69]. Although these diseases or conditions may occur independently in some patients, it should be considered that they may occur together in causal relationships or comorbidities in other patients, particularly in patients with chronic disease such as TMD. Among the patients with TMD pain, the number of poor sleepers was significantly higher in the TMD pain (76.8%) and myalgia groups (71.7%) than in the arthralgia group (54.8%) [70]. It has been found that myofascial pain in TMD is associated with elevated sleep fragmentation and increased frequency of RERA events [71]. The effect of TMD on sleep deterioration may differ, depending on the origin of TMD pain. However, further studies are needed to elucidate the relationship between the origin of TMD pain and sleep quality and their underlying mechanisms.
A significant relationship between OSA and TMD can be inferred from the high incidence of OSA in patients with TMD and vice versa. In the OPPERA (Orofacial Pain: Prospective Evaluation and Risk Assessment) cohort study on TMD and orofacial pain, loud snoring was a contributing factor to a high risk of OSA in patients with TMD [72]. However, few studies have examined the relationship between TMD and snoring as a subtype of SRBD, and there are only a few papers on OSA and TMD. In a population-based cohort of adults without TMD at baseline, baseline OSA signs/symptoms of the participants were associated with the incidence of first-onset TMD [73]. In an earlier study using polysomnography, out of 87 adults with mild or moderate OSA, 32 (36.8%) had TMD according to the research diagnostic criteria for TMD (RDC/TMD) [74]. Among 53 patients with myofascial TMD pain diagnosed using the RDC/TMD, 28% had OSA on polysomnography [74]. The effect of snoring on orofacial pain is not clear, with very few studies on this topic [75]. Only a few papers have been published on the relationship between SRBD and TMD. The relationship between these elements is still controversial, and further studies are needed.
The immediate effects of OSA on the body are persistent sympathetic excitation, oxyhemoglobin desaturation, blood pressure and heart rate fluctuations, cortical arousal, and sleep fragmentation [76]. The long-term effects of OSA may include various cardiovascular diseases, systemic hypertension, neurocognitive impairment, metabolic syndrome, morbidities, sociopsychological impairment, and chronic pain conditions [77,78]. The possible mechanisms by which SRBD may contribute to pain over time include pain amplification with decreasing functioning of the pain inhibitory systems and peripheral and central sensitizations [79]. A close relationship between sleep, pain, and central sensitization has been reported [80]. Sleep bruxism is a rhythmic masticatory muscle activity during sleep that is weakly correlated with certain parameters of OSA [81]. Sleep bruxism may ultimately increase myofascial pain in patients with TMD [82]. With regard to OSA and repeated facial movements, lack of adequate rest between muscle activities can lead to overload of related muscles and/or the TMJ. Moreover, SRBD-induced systemic sympathetic hyperactivity, increased inflammatory response, and secondary socio-psychological damage are highly likely outcomes. Further studies are required to investigate the mechanisms underlying the relationship between SRBD and TMD or the co-factors shared by two diseases such as psychological impairment and sleep bruxism.
Study limitations
This study aimed to determine the relationship between SRBDs and TMD, with a particular focus on snoring and OSA. However, there are insufficient statistically relevant data to draw specific conclusions regarding this relationship, and there is still a lack of controlled clinical trials or multicenter studies and randomized controlled trials on this issue. Since there are few original articles on the relationship between SRBD or TMD, it is difficult to determine the exact negative effects and underlying mechanisms of SRBD in patients with TMD or the negative effects and underlying mechanisms of TMD in those with SB. Moreover, co-occurrence of SRBDs, such as snoring and OSA and TMD, and other comorbidities, still needs further explanation.
Conclusions
In this narrative review, the epidemiology, etiology, and pathophysiology of SRBDs, particularly snoring and OSA, including latest research trends, were reviewed. Furthermore, recent research trends and knowledge on the relationship between SRBD and TMD were investigated. In particular, the pathogenesis of OSA involves not only anatomical causes involving the upper airways but also non-anatomical mechanisms such as function of the muscles of the upper airways, arousal threshold, and loop gain. OSA in patients with SRBDs may co-occur with TMD through several mechanisms. In future, studies should examine the specific relationship between SRBDs and TMD.
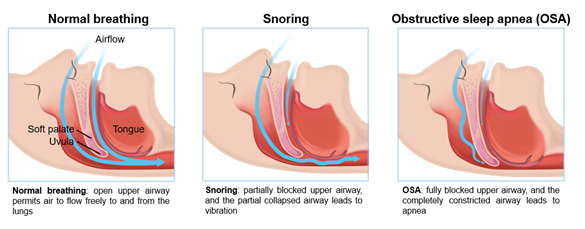
Figure 1: Schematic of snoring and obstructive sleep apnea compared to normal breathing
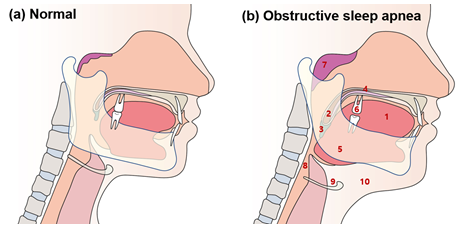
Figure 2: Typical anatomical features of obstructive sleep apnea in the orofacial area
Typical anatomical features of obstructive sleep apnea (OSA) in orofacial area are: (1) an abnormally enlarged tongue; (2) the long soft palate and/or tonsillar enlargement; (3) an extended uvula; (4) high palatal vault; (5) posteriorly positioned mandible (retrognathia) or small-sized mandible; (6) loss of normal occlusion; (7) adenoid hypertrophy; (8) reduced pharyngeal upper airway space; (9) the increased distance between the mandible and the hyoid bone; and (10) increased neck fat deposition surrounding the upper airway.
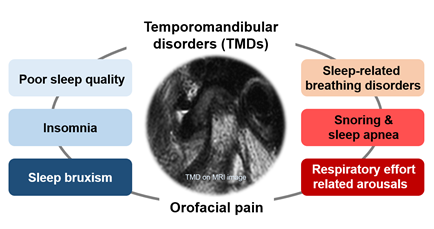
Figure 3: Temporomandibular disorder and sleep problems
Acknowledgments
None.
Author contributions
Conceptualization: Y.-H. Methodology: Y.-H. L.; resources: Y.-H. L. Writing -original draft preparation: Y.-H. L. writing, review, and editing: Y.-H. L.; supervision, Y.-H. L. The authors have read and agreed to the published version of the manuscript.
Data availability
The data supporting the findings of this study are available from the corresponding author, Y-. H.L. upon request.
Funding details
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (No. NRF-2020R1F1A1070072) and Kyung Hee University in 2021 (KHU-20211863).
Competing Interests Statement
The author declares no conflicts of interest.
Ethics declarations
The requirement for informed consent was waived by the institutional review board of Kyung Hee University Dental Hospital because of the study design.
References
- Alsaadi SM. The bidirectional relationship between pain intensity and sleep disturbance/quality in patients with low back pain. Clin J Pain 30 (2014): 755-765.
- Memon J, Manganaro SN in StatPearls (StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., 2022).
- Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord 16 (2015): 25-34.
- Mehrtash M, Bakker JP, Ayas N. Predictors of Continuous Positive Airway Pressure Adherence in Patients with Obstructive Sleep Apnea. Lung 197 (2019): 115-121.
- Esteller E. Clinical Practice Guideline recommendations on examination of the upper airway for adults with suspected obstructive sleep apnoea-hypopnoea syndrome. Acta Otorrinolaringol Esp (Engl Ed) 70 (2019): 364-372.
- Lee LA. Snoring Sounds Predict Obstruction Sites and Surgical Response in Patients with Obstructive Sleep Apnea Hypopnea Syndrome. Scientific Reports 6 (2016): 30629.
- Sowho M, Sgambati F, Guzman MA. Snoring: a source of noise pollution and sleep apnea predictor. Sleep 43 (2020).
- Merrill, R. L. Temporomandibular disorder pain and dental treatment of obstructive sleep apnea. Dent Clin North Am 56 (2012): 415-431.
- Michalek-Zrabkowska M. The Relationship between Simple Snoring and Sleep Bruxism: A Polysomnographic Study. Int J Environ Res Public Health 17 (2020): 4589.
- Counter P, Wilson JA. The management of simple snoring. Sleep Med Rev 8 (2004): 433-441.
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest 146 (2014): 1387-1394.
- Ohayon MM, Guilleminault C, Priest RG. Snoring and breathing pauses during sleep: telephone interview survey of a United Kingdom population sample. BMJ 314 (1997): 860-863.
- Chuang LP. The gender difference of snore distribution and increased tendency to snore in women with menopausal syndrome: a general population study. Sleep Breath 21 (2017): 543-547.
- Stuck BA. Diagnosis and treatment of snoring in adults-S2k Guideline of the German Society of Otorhinolaryngology, Head and Neck Surgery. Sleep Breath 19 (2015): 135-148.
- Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med 162 (2000): 740-748.
- Romero-Corral A, Caples SM, Lopez-Jimenez F. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest 137 (2010): 711-719.
- Zammit GK, Lund S, Ghassibi J. Clinical polysomnography in the evaluation of snoring and sleep-related breathing disorders. Operative Techniques in Otolaryngology-Head and Neck Surgery 5 (1994): 221-227.
- Bonekat HW, Hardin KA. Severe upper airway obstruction during sleep. Clin Rev Allergy Immunol 25 (2003): 191-197.
- Torzsa P. Socio-demographic characteristics, health behaviour, co-morbidity and accidents in snorers: a population survey. Sleep Breath 15 (2011): 809-818.
- Babisch W, Ising H, Gallacher JE, et al. Traffic noise and cardiovascular risk: the Caerphilly and Speedwell studies, third phase--10-year follow up. Arch Environ Health 54 (1999): 210-216.
- Taylor C. Snoring severity is associated with carotid vascular remodeling in young adults with overweight and obesity. Sleep Health 7 (2021): 161-167.
- Somers VK, Dyken ME, Mark AL, et al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328 (1993): 303-307.
- Haug SR, Heyeraas KJ. Modulation of dental inflammation by the sympathetic nervous system. J Dent Res 85 (2006): 488-495.
- Berry RB. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8 (2012): 597-619.
- Ferreira CB, Schoorlemmer GH, Rocha AA. Increased sympathetic responses induced by chronic obstructive sleep apnea are caused by sleep fragmentation. J Appl Physiol 129 (2020): 163-172.
- Tsara V, Amfilochiou A, Papagrigorakis MJ. Guidelines for diagnosis and treatment of sleep-related breathing disorders in adults and children. Definition and classification of sleep related breathing disorders in adults: different types and indications for sleep studies (Part 1). Hippokratia 13 (2009): 187-191.
- Goyal M, Johnson J. Obstructive Sleep Apnea Diagnosis and Management. Mo Med 114 (2017): 120-124.
- Rey de Castro J, Huamaní C, Escobar-Córdoba F, et al. Clinical factors associated with extreme sleep apnoea [AHI>100 events per hour] in Peruvian patients: A case-control study-A preliminary report. Sleep Sci 8 (2015): 31-35.
- Lee W, Nagubadi S, Kryger MH, et al. Epidemiology of Obstructive Sleep Apnea: a Population-based Perspective. Expert Rev Respir Med 2 (2008): 349-364.
- Haslam DW, James WP. Obesity. Lancet 366 (2005): 1197-1209.
- Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev 90 (2010): 47-112.
- Jean-Louis G, Zizi F, Clark LT, et al. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med 4 (2008): 261-272.
- Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci 8 (2011): 17-25.
- Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 7 (2015): 1311-1322.
- Asghari A, Mohammadi F. Is Apnea-Hypopnea Index a proper measure for Obstructive Sleep Apnea severity? Med J Islam Repub Iran 27 (2013): 161-162.
- Kainulainen S. Severity of Desaturations Reflects OSA-Related Daytime Sleepiness Better Than AHI. J Clin Sleep Med 15 (2019): 1135-1142.
- Polysomnography in patients with obstructive sleep apnea: an evidence-based analysis. Ont Health Technol Assess Ser 6 (2006): 1-38.
- Patil SP, Schneider H, Schwartz AR. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest 132 (2007): 325-337.
- El-Sherbini AM, Bediwy AS, El-Mitwalli A. Association between obstructive sleep apnea (OSA) and depression and the effect of continuous positive airway pressure (CPAP) treatment. Neuropsychiatr Dis Treat 7 (2011): 715-721.
- Tom C. et al. Correlations between Waist and Neck Circumferences and Obstructive Sleep Apnea Characteristics. Sleep Vigil 2 (2018): 111-118.
- Ha JG. The dimension of hyoid bone is independently associated with the severity of obstructive sleep apnea. PLoS One 8 (2013) e81590.
- Harvey R. Friedman tongue position and cone beam computed tomography in patients with obstructive sleep apnea. Laryngoscope Investig Otolaryngol 2 (2017): 320-324.
- Nuckton TJ, Glidden DV, Browner WS, et al. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep 29 (2006): 903-908.
- Amra B. Comparison of Berlin Questionnaire, STOP-Bang, and Epworth Sleepiness Scale for Diagnosing Obstructive Sleep Apnea in Persian Patients. Int J Prev Med 9 (2018): 28.
- Rosenthal LD, Dolan DC. The Epworth sleepiness scale in the identification of obstructive sleep apnea. J Nerv Ment Dis 196 (2008): 429-431.
- Cheng JX. Rapid eye movement sleep and slow wave sleep rebounded and related factors during positive airway pressure therapy. Scientific Reports 11 (2021): 7599.
- Osman AM, Carter SG, Carberry JC, et al. Obstructive sleep apnea: current perspectives. Nat Sci Sleep 10 (2018): 21-34.
- Zinchuk AV, Gentry MJ, Concato J, et al. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med Rev 35 (2017): 113-123.
- Genta PR. Upper airway collapsibility is associated with obesity and hyoid position. Sleep 37 (2014): 1673-1678.
- Schwartz AR. Obesity and upper airway control during sleep. J Appl Physiol 108 (2010): 430-435.
- Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 188 (2013): 996-1004.
- Dieltjens M, Vanderveken O. Oral Appliances in Obstructive Sleep Apnea. Healthcare (Basel) 7 (2019): 201978.
- Sutherland K, Cistulli PA. Oral Appliance Therapy for Obstructive Sleep Apnoea: State of the Art. J Clin Med 8 (2019): 781-795.
- Gray EL, McKenzie DK, Eckert DJ. Obstructive Sleep Apnea without Obesity Is Common and Difficult to Treat: Evidence for a Distinct Pathophysiological Phenotype. J Clin Sleep Med 13 (2017): 81-88.
- Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir Physiol Neurobiol 160 (2008): 1-7.
- Amatoury J. New insights into the timing and potential mechanisms of respiratory-induced cortical arousals in obstructive sleep apnea. Sleep 41 (2018): 654-661.
- Urahama R. Occurrence of Cortical Arousal at Recovery from Respiratory Disturbances during Deep Propofol Sedation. Int J Environ Res Public Health 16 (2019): 896-912.
- Eckert DJ. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 120 (2011): 505-514.
- Bahr K. Intensity of respiratory cortical arousals is a distinct pathophysiologic feature and is associated with disease severity in obstructive sleep apnea patients. Brain Sci 11 (2021): 282-298.
- Burgess KR. New insights from the measurement of loop gain in obstructive sleep apnoea. J Physiol 590 (2012): 1781-1782.
- Li Y. The effect of upper airway surgery on loop gain in Obstructive Sleep Apnea. J Clin Sleep Med 15 (2019): 907-913.
- Deacon-Diaz N, Malhotra A. Inherent vs. Induced Loop Gain Abnormalities in Obstructive Sleep Apnea. Front Neurol 9 (2018): 896.
- Selim B, Ramar K. Sleep-Related Breathing Disorders: When CPAP Is Not Enough. Neurotherapeutics 18 (2021): 81-90.
- Lee YH, Lee KM, Auh QS. MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury. Journal of Clinical Medicine 10 (2021): 1404.
- List T, Jensen RH. Temporomandibular disorders: Old ideas and new concepts. Cephalalgia 37 (2017): 692-704.
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain 14 (2013): 1539-1552.
- Schiffman E. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache 28 (2014): 6-27.
- Lee YH, Auh QS, An JS, et al. Poorer sleep quality in patients with chronic temporomandibular disorders compared to healthy controls. BMC Musculoskelet Disord 23 (2022): 246.
- Smith MT. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep 32 (2009): 779-790.
- Lee YH, Auh QS. Comparison of sleep quality deterioration by subgroup of painful temporomandibular disorder based on diagnostic criteria for temporomandibular disorders. Sci Rep 12 (2022): 9026.
- Dubrovsky B. Polysomnographic investigation of sleep and respiratory parameters in women with temporomandibular pain disorders. J Clin Sleep Med 10 (2014): 195-201.
- Slade GD. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain 12 (2011): T12-26.
- Sanders AE. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. J Dent Res 92 (2013): 70s-77s.
- Cunali PA. Prevalence of temporomandibular disorders in obstructive sleep apnea patients referred for oral appliance therapy. J Orofac Pain 23 (2009): 339-344.
- Dal Fabbro C. Orofacial Pain and Snoring/Obstructive Sleep Apnea in Individuals with Head and Neck Cancer: A Critical Review. J Oral Facial Pain Headache 36 (2022): 85-102.
- Taylor KS. Arousal From Sleep and Sympathetic Excitation During Wakefulness. Hypertension 68 (2016): 1467-1474.
- Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med 6 (2012): 557-566.
- Aytekin E. Chronic widespread musculoskeletal pain in patients with obstructive sleep apnea syndrome and the relationship between sleep disorder and pain level, quality of life, and disability. J Phys Ther Sci 27 (2015): 2951-2954.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states- maybe it is all in their head. Best Pract Res Clin Rheumatol 25 (2011): 141-154.
- Campbell CM. Sleep, Pain Catastrophizing, and Central Sensitization in Knee Osteoarthritis Patients with and without Insomnia. Arthritis Care Res (Hoboken) 67 (2015): 1387-1396.
- Saito M. Weak association between sleep bruxism and obstructive sleep apnea. A sleep laboratory study. Sleep Breath 20 (2016): 703-709.
- Muzalev K, Lobbezoo F, Janal MN, et al. Interepisode Sleep Bruxism Intervals and Myofascial Face Pain. Sleep 40 (2017): 56-71.
