Helicobacter Pylori Infection Triggers PERK-Associated Survivin Loss in Gastric Tissue Samples and Cell Lines
Article Information
Paula Díaz1,2, Alejandra Román2, Gonzalo Carrasco-Aviño2, Andrés Rodríguez2,4, Alejandro H Corvalán3,4,5, Sergio Lavandero2,4,6* and Andrew FG Quest1,2,4*
1Laboratory of Cellular Communication, Center for the Study of Exercise, Metabolism and Cancer (CEMC), Program of Cell and Molecular Biology, Institute of Biomedical Sciences (ICBM), Faculty of Medicine, Universidad de Chile, Santiago, Chile
2Advanced Center for Chronic Diseases (ACCDiS), Faculty of Chemical and Pharmaceutical Sciences & Faculty of Medicine, Universidad de Chile, Santiago, Chile
3Advanced Center for Chronic Diseases (ACCDiS), Faculty of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
4Corporación Centro de Estudios Científicos de las Enfermedades Crónicas (CECEC), Santiago, Chile
5Laboratory of Oncology, Department of Hematology and Oncology, Pontificia Universidad Católica de Chile, Santiago, Chile
6Department of Internal Medicine (Cardiology Division), University of Texas Southwestern Medical Center, Dallas, TX, USA
*Corresponding Authors: Dr. Andrew Quest, Center for the Study of Exercise, Metabolism and Cancer (CEMC), Facultad de Medicina, Universidad de Chile, Av. Independencia 1027, Santiago 8380453, Chile
Dr. Sergio Lavandero, Advanced Center for Chronic Diseases (ACCDiS), Faculty of Chemical and Pharmaceutical Sciences & Faculty of Medicine, Universidad de Chile, Santiago 8380492, Chile
Received: 25 November 2020; Accepted: 18 December 2020; Published: 08 January 2021
Citation:
Paula Díaz, Alejandra Román, Gonzalo Carrasco-Aviño, Andrés Rodríguez, Alejandro H Corvalán, Sergio Lavandero and Andrew FG Quest. Helicobacter Pylori Infection Triggers PERK-Associated Survivin Loss in Gastric Tissue Samples and Cell Lines. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 063-082.
View / Download Pdf Share at FacebookAbstract
Infection by Helicobacter pylori (Hp) is the main risk factor associated with the development and progression of precancerous lesions to gastric cancer. Protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) is activated by endoplasmic reticulum (ER) stress, and ER stress-induced apoptotic cell death has been associated with Hp infection. Survivin, an inhibitor of apoptosis, is downregulated in the mucosa of Hp infected subjects and loss of survivin in gastric cells correlates with reduced viability. Here, we determined whether Hp-induced changes in PERK contribute to the loss of survivin, previously associated with the genesis of gastric cancer precancerous lesions. Our results show that PERK is activated in the early stages of Hp infection in the human gastric mucosa affected by gastritis and PERK activation coincided with reduced survivin levels compared with Hp-negative gastritis. Upon Hp infection in vitro, PERK silencing restored survivin abundance and its activity increased survivin loss, in parallel with a partial downstream activation of the α- subunit of eukaryotic initiation factor-2 (eIF2α). Our results suggest a novel mechanism by which Hp-stimulated PERK reduces gastric cell viability/proliferation by downregulating survivin and is likely to favour the genesis of gastric cancer precancerous lesions.
Supplementary-file
Keywords
Helicobacter pylori; PERK; survivin; ER stress; precancerous gastric lesion; gastritis; gastric cancer
Helicobacter pylori articles; PERK articles; survivin articles; ER stress articles; precancerous gastric lesion articles; gastritis articles; gastric cancer articles
Helicobacter pylori articles Helicobacter pylori Research articles Helicobacter pylori review articles Helicobacter pylori PubMed articles Helicobacter pylori PubMed Central articles Helicobacter pylori 2023 articles Helicobacter pylori 2024 articles Helicobacter pylori Scopus articles Helicobacter pylori impact factor journals Helicobacter pylori Scopus journals Helicobacter pylori PubMed journals Helicobacter pylori medical journals Helicobacter pylori free journals Helicobacter pylori best journals Helicobacter pylori top journals Helicobacter pylori free medical journals Helicobacter pylori famous journals Helicobacter pylori Google Scholar indexed journals PERK articles PERK Research articles PERK review articles PERK PubMed articles PERK PubMed Central articles PERK 2023 articles PERK 2024 articles PERK Scopus articles PERK impact factor journals PERK Scopus journals PERK PubMed journals PERK medical journals PERK free journals PERK best journals PERK top journals PERK free medical journals PERK famous journals PERK Google Scholar indexed journals survivin articles survivin Research articles survivin review articles survivin PubMed articles survivin PubMed Central articles survivin 2023 articles survivin 2024 articles survivin Scopus articles survivin impact factor journals survivin Scopus journals survivin PubMed journals survivin medical journals survivin free journals survivin best journals survivin top journals survivin free medical journals survivin famous journals survivin Google Scholar indexed journals ER stress articles ER stress Research articles ER stress review articles ER stress PubMed articles ER stress PubMed Central articles ER stress 2023 articles ER stress 2024 articles ER stress Scopus articles ER stress impact factor journals ER stress Scopus journals ER stress PubMed journals ER stress medical journals ER stress free journals ER stress best journals ER stress top journals ER stress free medical journals ER stress famous journals ER stress Google Scholar indexed journals precancerous gastric lesion articles precancerous gastric lesion Research articles precancerous gastric lesion review articles precancerous gastric lesion PubMed articles precancerous gastric lesion PubMed Central articles precancerous gastric lesion 2023 articles precancerous gastric lesion 2024 articles precancerous gastric lesion Scopus articles precancerous gastric lesion impact factor journals precancerous gastric lesion Scopus journals precancerous gastric lesion PubMed journals precancerous gastric lesion medical journals precancerous gastric lesion free journals precancerous gastric lesion best journals precancerous gastric lesion top journals precancerous gastric lesion free medical journals precancerous gastric lesion famous journals precancerous gastric lesion Google Scholar indexed journals gastritis articles gastritis Research articles gastritis review articles gastritis PubMed articles gastritis PubMed Central articles gastritis 2023 articles gastritis 2024 articles gastritis Scopus articles gastritis impact factor journals gastritis Scopus journals gastritis PubMed journals gastritis medical journals gastritis free journals gastritis best journals gastritis top journals gastritis free medical journals gastritis famous journals gastritis Google Scholar indexed journals gastric cancer articles gastric cancer Research articles gastric cancer review articles gastric cancer PubMed articles gastric cancer PubMed Central articles gastric cancer 2023 articles gastric cancer 2024 articles gastric cancer Scopus articles gastric cancer impact factor journals gastric cancer Scopus journals gastric cancer PubMed journals gastric cancer medical journals gastric cancer free journals gastric cancer best journals gastric cancer top journals gastric cancer free medical journals gastric cancer famous journals gastric cancer Google Scholar indexed journals intestinal-type adenocarcinoma articles intestinal-type adenocarcinoma Research articles intestinal-type adenocarcinoma review articles intestinal-type adenocarcinoma PubMed articles intestinal-type adenocarcinoma PubMed Central articles intestinal-type adenocarcinoma 2023 articles intestinal-type adenocarcinoma 2024 articles intestinal-type adenocarcinoma Scopus articles intestinal-type adenocarcinoma impact factor journals intestinal-type adenocarcinoma Scopus journals intestinal-type adenocarcinoma PubMed journals intestinal-type adenocarcinoma medical journals intestinal-type adenocarcinoma free journals intestinal-type adenocarcinoma best journals intestinal-type adenocarcinoma top journals intestinal-type adenocarcinoma free medical journals intestinal-type adenocarcinoma famous journals intestinal-type adenocarcinoma Google Scholar indexed journals intestinal metaplasia articles intestinal metaplasia Research articles intestinal metaplasia review articles intestinal metaplasia PubMed articles intestinal metaplasia PubMed Central articles intestinal metaplasia 2023 articles intestinal metaplasia 2024 articles intestinal metaplasia Scopus articles intestinal metaplasia impact factor journals intestinal metaplasia Scopus journals intestinal metaplasia PubMed journals intestinal metaplasia medical journals intestinal metaplasia free journals intestinal metaplasia best journals intestinal metaplasia top journals intestinal metaplasia free medical journals intestinal metaplasia famous journals intestinal metaplasia Google Scholar indexed journals dysplasia articles dysplasia Research articles dysplasia review articles dysplasia PubMed articles dysplasia PubMed Central articles dysplasia 2023 articles dysplasia 2024 articles dysplasia Scopus articles dysplasia impact factor journals dysplasia Scopus journals dysplasia PubMed journals dysplasia medical journals dysplasia free journals dysplasia best journals dysplasia top journals dysplasia free medical journals dysplasia famous journals dysplasia Google Scholar indexed journals
Article Details
1. Introduction
Gastric cancer constitutes the third leading cause of cancer-related mortality worldwide [1]. This high mortality rate is often related to a late diagnosis, due to the lack of early signs or symptoms [2]. Infection by Helicobacter pylori (Hp), a Gram-negative bacterium that colonises the gastric mucosa [3], constitutes the main risk factor associated with the development of gastric cancer. On average, Hp colonizes the gastric epithelium of about 50% of the world population [4]. The intestinal-type adenocarcinoma is the most frequent type of gastric cancer [5]. The latter is preceded by a prolonged multistep process termed precancerous gastric lesions [6-8]. These precancerous lesions begin with the transition from normal gastric mucosa to chronic non-atrophic gastritis, triggered commonly by Hp infection, which then leads to atrophic gastritis, intestinal metaplasia, and finally to dysplasia and gastric cancer [6-9]. Thus, Hp infection is particularly relevant during early stages of gastric carcinogenesis since eradication with antibiotics considerably reduces the presence of gastric precancerous lesions [10] until the intestinal metaplasia stage; however, antibiotics are ineffective once the disease has progressed beyond this stage [11, 12].
Hp infection triggers an inflammatory response indirectly mediated by cytokines, which facilitate the induction of chronic inflammation [13], or directly in host gastric cells through the action of bacterial virulence factors [14-16]. In addition, Hp chronic infection induces cellular adaptive mechanisms, such as apoptosis of gastric cells and reduced epithelial turnover leading to atrophy [17, 18], compensatory increases in cell proliferation [19, 20], phenotypic changes [21, 22] and endoplasmic reticulum (ER) stress-induced apoptotic cell death [23, 24].
The accumulation of unfolded and/or misfolded proteins within the ER induces ER stress, which can be resolved by an adaptive mechanism termed the unfolded protein response (UPR) [25]. Amongst ER stress sensors, the serine/threonine protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), is a protein whose activity is regulated by protein misfolding within the ER [26]. Upon perturbation of ER homeostasis and accumulation of unfolded/misfolded proteins, PERK is activated undergoing simultaneous dimerisation/oligomerisation and autophosphorylation [25]. The PERK canonical pathway inhibits global protein translation, hence the number of proteins entering the ER is reduced [27]. However, failure to resolve ER stress induces the UPR, which initiates stress signalling pathways including those involved in cell death [28-30]. Particularly, Hp infection triggers ER stress [23] and activates the UPR [23, 31]. In Hp-infected gastric mucosa, BiP/GRP78 (binding immunoglobulin protein), a marker of ER stress, is increased compared with healthy Hp-negative subjects [23].
We have previously shown that survivin, a member of the inhibitor of apoptosis family of proteins [32], which is over-expressed in many human cancers [33], is down-regulated in the mucosa of Hp infected subjects [34]. In vitro, loss of survivin correlated with increased apoptosis and reduced viability in gastric cell lines [15, 34]. Loss of survivin due to Hp is predicted to disrupt gastric mucosa homeostasis [15, 34] which could then exacerbate signalling pathways that favour the progression of precancerous lesions. Evidence suggests that Hp triggers ER stress [23] and activates the UPR [23, 31] leading to gastric cell death; however, it remains unclear whether these cellular mechanisms may be connected to Hp-induced down-regulation of survivin. Here we investigated whether Hp-induced changes in the ER stress sensor protein PERK may contribute to the loss of survivin, previously associated with gastric cell death. These findings represent the first molecular link that connects ER stress-triggered by Hp infection to gastric cell death and the genesis of gastric cancer precancerous lesions.
2. Materials and Methods
2.1 Tissue Samples
Gastric biopsy specimens from corpus and antrum of 50 subjects who underwent upper endoscopy between 2010 and 2012 at the CRS - San Rafael, SSMSO, Santiago were analysed. Written informed consent, approved by the Ethics Committee of the Pontificia Universidad Católica de Chile (project #14-280; date 4 June 2015) and the Ethics Committee of the Faculty of Medicine, Universidad de Chile (project #046-2017; date 9 May 2017), was obtained from each subject. The study was conducted in accordance with the rules of the Helsinki declaration. Gastric precancerous lesions were classified based on the Operative Link on Gastritis Assessment (OLGA) [35], a histological assessment of random biopsies taken from antrum and corpus areas of the stomach according to the Sydney protocol [36]. At least four sites were sampled from the stomach during upper endoscopy (two antral and two corpus). The OLGA scoring standard was used to grade and stage chronic gastric inflammation, gastric atrophy and intestinal metaplasia. The analysis of corpus and antrum biopsies corresponded to sections from early stages of gastritis (OLGA 0-II), clinically grouped in Hp-positive and Hp-negative cases. On average, intestinal metaplasia was undetectable or less than 30% in all samples. Hp status was evaluated by the urease test [37], Giemsa staining [38] and immunohistochemistry (see below).
Biopsies were collected at room temperature during the endoscopic procedure. Samples were fixed in zinc-formalin at 4°C for 24 h and then transferred to phosphate-buffered saline pH 7.2 and kept at 4°C. After standard histological processing, gastric tissue biopsies were embedded in paraffin and 3mm-thick sections were obtained, subsequently deparaffinised, rehydrated, and routinely stained with haematoxylin-eosin for histopathological evaluation.
2.2 Immunohistochemistry analysis
We used Resource Identification Portal (RRID) to allow research resource identification where possible. The protein abundance of PERK (1:500; Cell Signaling Technology Cat# 3192, RRID:AB_2095847), phospho-PERK (P-PERK(Thr982)) (1:500; Abcam Cat# ab192591, RRID:AB_2728666), and survivin (1:300; R and D Systems Cat# AF886, RRID:AB_355684) was detected using the avidin-biotin-peroxidase complex method (VECTASTAIN® Universal HRP Kit; Vector Laboratories Cat# PK-7800, RRID:AB_2336829) in gastric biopsy sections and the 3,3'-diaminobenzidine (DAB)-Substrate Chromogen (Dako #K3468). The presence of Hp in samples was detected with an Hp-specific polyclonal antibody (Dako #B0471). All sections were counterstained with haematoxylin. Quantification and analysis of PERK, P-PERK(Thr982) and survivin-Q-score (intensity multiplied by percentage of positively stained cells) was performed by two independent pathologists who were unaware of the clinical data and the objectives of the study (G.C. and A.R.).
2.3 Cell lines, strains, and culture conditions
The gastric adenocarcinoma cell line AGS, obtained from the American Tissue Culture Collection (ATCC Cat# CRL-1739, RRID:CVCL_0139), and the non-transformed gastric epithelial cell line GES-1 (kindly provided by Dr. Armando Rojas, Universidad Católica del Maule, Chile) were cultured in Roswell Park Memorial Institute medium (RPMI 1640 medium; Thermo Fisher Scientific) supplemented with 10% Fetal Bovine Serum (Biological Industries) and antibiotics (10,000 U/mL penicillin, 10 mg/mL streptomycin), at 37°C in a humidified atmosphere supplemented with 5% CO2. The completely sequenced Helicobacter pylori (Hp) strain 26695 [KE26695] (ATCC Cat#700392) was used. Hp was routinely grown on trypticase soy agar (TSA) plates supplemented with 5% horse serum (Biological Industries), the culture supplement Vitox (Oxoid), and the antibiotic supplement Dent (Oxoid) for 24 h at 37°C in a humidified atmosphere supplemented with 5% CO2 [34].
2.4 Infection of gastric cells with Helicobacter pylori
GES-1 and/or AGS cells were infected with a multiplicity of infection (MOI) of 100 (bacteria:cell ratio of 100:1) for 4 and 24 h as previously described by Valenzuela et al. [15, 34]. For infection experiments 8x105 cells were seeded onto a 60-mm tissue culture plate in culture medium without antibiotics for 24 h and then infected with Hp.
2.5 siRNA transfection
GES-1 and AGS cells were transfected with 100 nM SignalSilence® PERK siRNA I (siRNA PERK) (Cell Signaling Technology #9024) or 100 nM non-specific Control siRNA (Cnt siRNA) (Unconjugated; Cell Signaling Technology #6568) 16 h before Hp infection using the Lipofectamine RNAi Max reagent (Thermo Scientific) following the manufacturer’s instructions.
2.6 Inhibition of PERK activity
GES-1 and AGS cells were pre-incubated with 25 mM GSK2606414 (Tocris Bioscience #5107) [39, 40], a selective PERK inhibitor, 1 h before Hp infection.
2.7 Immunoblotting analysis
AGS or GES-1 cells were lysed in radioimmunoprecipitation assay buffer (50 mM Tris-HCl pH 8.0; 150 mM NaCl, 1% NP-40 (IGEPAL #CA-630), 0.5%, sodium deoxycholate and 0.1% sodium dodecyl sulphate (SDS)) with protease and phosphatase inhibitors and proteins (50 mg/lane) were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes [16]. Blots were blocked for 1 h at room temperature using 3% bovine serum albumin (Rockland) in Tris-buffered saline (TBS)-Tween. Membranes were then incubated in primary antibody diluted in 3% bovine serum albumin in TBS-Tween. Protein abundance of PERK (1:1000; Cell Signaling Technology Cat# 3192, RRID:AB_2095847), phospho-PERK (P-PERK(Thr982)) (1:1000; Abcam Cat# ab192591, RRID:AB_2728666), survivin (1:3000; R and D Systems Cat# AF886, RRID:AB_355684), phospho-eIF2a (P-eIF2a(Ser51)) (1:1000; Abcam Cat# ab32157, RRID:AB_732117), eIF2a (1:1000; Santa Cruz Biotechnology Cat# sc-133132, RRID:AB_1562699) and PUMA a/b (1:1000; Santa Cruz Biotechnology Cat# sc-374223, RRID:AB_10987708) was determined in GES-1 and AGS cells. Horseradish peroxidase conjugated secondary anti-rabbit and anti-mouse antibodies were used (Rockland) and immunolabeling was visualized with enhanced chemiluminescence detection solution (Thermo Scientific). Densitometry was performed using ImageJ software (http://rsbweb.nih.gov/ij/; National Institutes of Health). Target protein abundance in each individual lane was normalized to b-actin (1:10,000; Sigma-Aldrich Cat# A5316, RRID:AB_476743) or Hsp90 (1:5000; Santa Cruz Biotechnology Cat# sc-13119, RRID:AB_675659).
2.8 BrdU incorporation assay
Gastric cells were plated on 96-well plates at a density of 5x103 cells/well. Cell proliferation was detected after 24 h of Hp infection in uninfected and infected cells by measuring 5-bromo-2'-deoxyuridine (BrdU) incorporation using a colorimetric BrdU Cell Proliferation Assay Kit (BioVision, Inc. #K306) according to the manufacturer’s instructions.
2.9 Viability assay
Gastric cells were plated on 96-well plates at a density of 5x103 cells/well. Cell viability was evaluated after 24 h of incubation in uninfected and Hp-infected cells by the MTS assay (Promega #G5421) according to the manufacturer’s instructions.
2.10 Statistical analysis
Clinical data were expressed as mean ± standard error of the mean (SEM). Other data were expressed as the mean + SEM of at least 3 independent experiments. Statistical analysis was carried out with GraphPad Prism software version 5.0 (San Diego, CA, USA). To determine whether data distribution was normal, a Shapiro-Wilk test was initially performed. Clinical sample analyses were performed using Mann-Whitney test. Immunoblotting statistical analyses, using one-way ANOVA (or non-parametric) test with Bonferroni post-hoc test and assay data were analysed using two-way ANOVA. Statistical significance was defined as p<0.05.
3. Results
3.1 PERK activity increased in human gastric tissue samples infected with Helicobacter pylori
Hp infection is relevant during the early stages of gastric carcinogenesis [10], particularly during gastritis. Thus, we examined stomach corpus and antrum biopsy sections from OLGA 0-II stage subjects, an equivalent to the early stages of gastritis, clinically grouped in Hp-positive and Hp-negative cases. PERK protein was detected in gastric glandular cells from corpus and antrum biopsy sections from subjects in OLGA 0-II stages (Figure 1). Although PERK staining varied in intensity between samples, total PERK levels remained unchanged between Hp-infected and non-infected individuals (Figure 1A,B). Representative PERK immunostaining images are shown in Figure 1E. P-PERK (Thr982), used as a marker of PERK activation, showed a significant increase in subjects with Hp-associated gastritis in both antrum and corpus, compared with Hp-negative individuals (Figure 1C,D). P-PERK (Thr982) staining localized to the cytoplasm of gastric glandular cells and also to the surrounding mesenchymal tissue in Hp positive-OLGA 0-II gastric biopsy sections (Figure 1E). Whereas total PERK protein abundance remained independent of Hp infection, PERK activity was markedly increased in gastric tissue samples from early stages of Hp-associated gastritis. Given our previously published studies linking Hp-induced gastritis to loss of the inhibitor of apoptosis survivin, we sought to determine whether PERK activity and/or abundance changed in Hp-infected gastric cells in vitro and whether these changes might be linked to the loss of survivin.
3.2 Total PERK abundance remained unchanged following Helicobacter pylori infection of gastric cells
To determine whether Hp infection induces changes in total PERK abundance in vitro, we infected gastric cell lines GES-1 and AGS with the wild-type Hp strain 26695 for 24 h, at a multiplicity of infection (MOI) of 100. Immunoblotting analysis of cell lysates revealed that PERK abundance remained unaltered following Hp infection for 4 and 24 h, compared with uninfected controls for 24 h (Figure 2A,B). Silencing of PERK resulted in a significant reduction in PERK protein levels by 80% to 86% in GES-1 and AGS cells after 24 h of Hp infection, respectively (Figure 2A,B). In addition, PERK silencing significantly increased cell viability following Hp infection, by ~35% after 24 h (Suppl. Figure 1A). Pharmacological inhibition of PERK activity, using the PERK inhibitor GSK2606414, did not have an effect on PERK abundance after Hp infection for 24 h (Figure 2C,D). Thus, as was observed in the clinical samples, total PERK protein abundance was not affected by Hp infection in vitro.
3.3 PERK activity following Helicobacter pylori infection of gastric cells
Hp infection for 24 h induced a significant 3-fold increase in PERK activation in the non-transformed gastric cell line GES-1, which was then inhibited, by the PERK kinase activity inhibitor GSK2606414 (Figure 3A). PERK activation status in transformed gastric AGS cells remained unchanged after 24 h Hp infection in (Figure 3B), though PERK activity was then reduced (~50%) after treatment with GSK2606414 (Figure 3B) suggesting that, after 24 h, Hp infection does not induce PERK activation in AGS cells. However, time-course experiments evaluating PERK activation in AGS cells suggest that PERK activity is significantly higher at earlier times of infection. After 4 h of Hp infection PERK activity increased compared to uninfected cells, and this increment was partly inhibited by GSK2606414 (Suppl. Figure 2).
3.4 Survivin abundance decreased in human gastric tissue samples infected with Helicobacter pylori
ER stress-induced apoptotic cell death, via the PERK pathway, has been associated with Hp infection [23]. Moreover, studies from our laboratory linked loss of survivin in Hp infected cells to apoptosis [15, 34]. With this in mind, we hypothesized that Hp-induced changes in PERK may contribute to the loss of survivin in gastric cells. Therefore, we determined whether survivin abundance also changed in the same clinical cohort evaluated in Figure 1. Survivin staining was predominantly located to the cytoplasm of gastric glandular cells in corpus and antrum gastric biopsy sections from OLGA 0-II stages of gastritis, clinically grouped in Hp-positive and Hp-negative cases (Figure 4). Survivin levels decreased significantly in both the antrum and corpus of Hp-positive individuals, compared with individuals with Hp-negative gastritis (Figure 4A,B). Representative immunostaining images are shown in Figure 4C. Therefore, increased PERK activity in biopsy sections from OLGA 0-II stages of gastritis of Hp infected subjects coincides with down-regulation of survivin levels.
3.5 Helicobacter pylori-stimulated PERK activity down-regulated survivin abundance
Immunoblotting analysis of gastric cell lysates revealed that survivin was reduced 30% in GES-1 cells (Figure 5A), and decreased 50% in AGS cells (Figure 5B) after 24 h of Hp infection. This effect was reverted upon PERK silencing, where we detected a 21% and 50% recovery in survivin levels after 24 h Hp infection in GES-1 and AGS cells, respectively. Restoration of survivin levels by silencing PERK correlated with increased proliferation of AGS cells (Suppl. Figure 1B). The pharmacological inhibition of PERK activity caused a marked restoration of survivin abundance by increasing levels 23% in GES-1 cells (Figure 5C), and completely restoring survivin abundance in AGS cells (Figure 5D). In addition, activation of the PERK pathway initiates expression of pro-apoptotic BH3-only proteins [29], which control cellular homeostasis and also participate in mitochondria-dependent cell death [30]. Particularly, p53 upregulated modulator of apoptosis a/b (PUMAa/b) is a master regulator of apoptosis that inhibits anti-apoptotic Bcl-2 family members [41]. Preliminary data show that the abundance of PUMAa/b is increased after 24 h of Hp infection, but PERK silencing did not have an effect on PUMAa/b abundance in gastric cells infected with Hp (Suppl. Figure 3).
3.6 Activation of eIF2a partly contributed to the reduction in survivin protein levels
The PERK canonical pathway inhibits global protein translation by direct phosphorylation of the a-subunit of eukaryotic initiation factor (eIF2a). In response to eIF2a phosphorylation, the number of proteins entering the ER is reduced [27], but translation of other mRNAs, such as activating transcription factor 4 (ATF4) [42] and C/EBP-homologous protein (CHOP) increases, leading to cell death (49). Thus, we tested whether the activation of eIF2a via the PERK pathway is a potential mechanism linking PERK to the loss of survivin after Hp infection in gastric cells. Non-transformed GES-1 cells displayed significant eIF2a activation (~50% increase) 24 h after Hp infection, compared with control uninfected cells, which was partially reduced (32%) when PERK was silenced (Figure 6A). Inhibition of PERK activity during Hp infection for 24 h led to a mild but significant decrease in eIF2a activation (Figure 6B). Total eIF2a protein abundance remained unaffected by Hp infection for 24 h in GES-1 cells (Suppl. Figure 5A). On the contrary, the transformed gastric adenocarcinoma cell line AGS displayed eIF2a activation after 4 and 24 h Hp infection to a lesser extent compared with uninfected controls (Suppl. Figure 4A). This response was unaffected by either PERK silencing (Suppl. Figure 4A) or the pharmacological inhibition of PERK activity (Suppl. Figure 4B). Likewise, total eIF2a abundance was unchanged by Hp infection after 24 h (Suppl. Figure 5B). As in Figure 3B, where PERK activation remained unchanged, the reduced eIF2a downstream activation after Hp infection suggests that the tumour-derived AGS cells already display high ER stress levels.
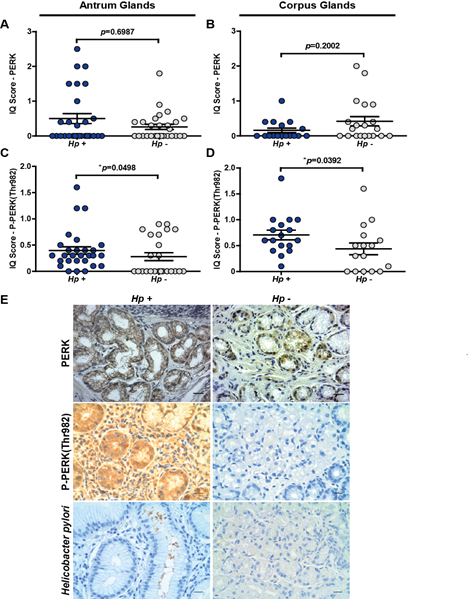
Figure 1: Immunohistochemical detection and analysis of PERK and P-PERK(Thr982) in gastric tissue samples of corpus and antrum from OLGA 0-II stages grouped in Hp-positive and Hp-negative individuals. (A) Semi-quantitative scoring (IQ) of PERK staining in antrum glands in Hp-positive and Hp-negative individuals; n=28, p=0.6987, mean ± SEM; (B) IQ score of PERK staining in corpus glands in Hp-positive and Hp-negative individuals; n=17, p=0.2002, mean ± SEM; (C) IQ score of P-PERK(Thr982) staining in antrum glands in Hp-positive and Hp-negative individuals; n=24, *p=0.0498, mean ± SEM; (D) IQ score of P-PERK(Thr982) staining in corpus glands in Hp-positive and Hp-negative individuals; n=16, *p=0.0392, mean ± SEM; (E) Representative PERK, P-PERK(Thr982) and Helicobacter pylori (Hp) immunostaining in gastric tissue. Immunostaining is visualised as a brown pigment. All samples were counterstained with haematoxylin (blue: nuclei). Scale bar: 50 mm.
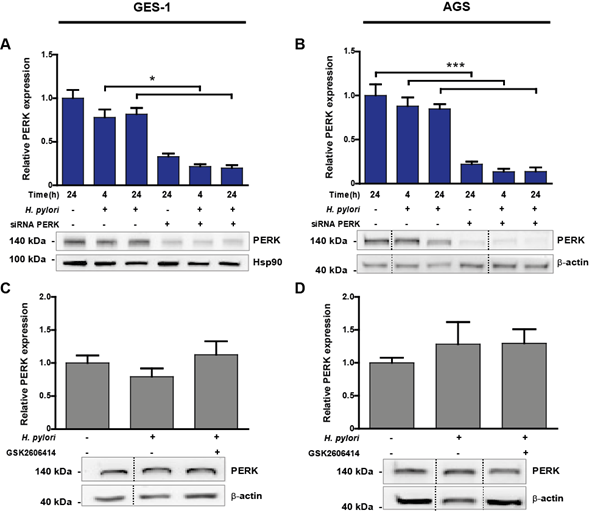
Figure 2: (A-B) Representative immunoblots with the corresponding graphic quantifications showing the mean relative protein abundance of PERK (normalised to b-actin or Hsp90 abundance) in uninfected (H. pylori -) and Hp-infected (H. pylori +) GES-1 (A) and AGS (B) cells. Gastric cells were infected with Hp for 4 and 24 h; in parallel, uninfected control cells were kept in medium for 24 h. PERK silencing (siRNA PERK) was achieved by transfecting GES-1 or AGS cells with 100 nM PERK siRNA 16 h before Hp infection. Non-specific siRNA (100 nM) was used as control (-). (A) n=5, *p<0.05; mean + SEM; (B) n=7, ***p<0.001; mean + SEM. (C-D) PERK protein abundance in uninfected (H. pylori -) and Hp-infected (H. pylori +) GES-1 (C) and AGS (D) cells after 24 h. Representative immunoblots with the corresponding graphic quantifications showing the mean relative protein abundance of PERK (normalised to b-actin abundance). GES-1 and AGS cells were pre-incubated with 25 mM GSK2606414, a selective PERK inhibitor, 1 h before Hp infection. (C) n=5; mean + SEM; (D) n=7; mean + SEM. Dotted lines indicate where lanes, from the same immunoblot, have been removed for clarity.
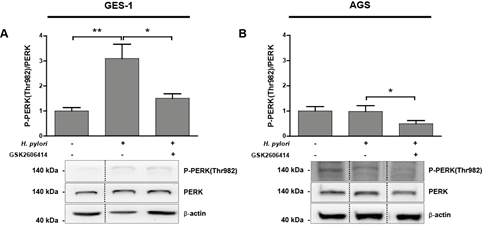
Figure 3: (A-B) P-PERK(Thr982) protein levels in uninfected (H. pylori -) and Hp infected (H. pylori +) GES-1 (A) and AGS (B) cells after 24 h. Representative immunoblots with the corresponding quantifications from several experiments showing P-PERK(Thr982)/PERK ratios. Values for P-PERK(Thr982) and PERK were normalised to b-actin abundance. GES-1 and AGS cells were pre-incubated for 1 h with 25 mM GSK2606414 before Hp infection. (A) n=5, **p<0.01, *p<0.05; mean + SEM; (B) n=4, *p<0.05; mean + SEM. Dotted lines indicate where lanes, from the same immunoblot, have been removed for clarity.
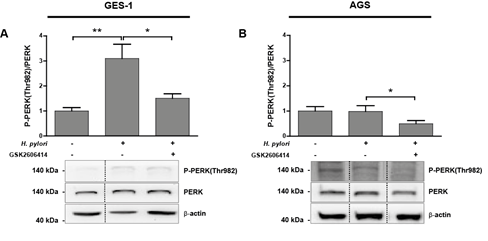
Figure 4: Immunohistochemical detection and analysis of survivin in gastric tissue samples from corpus and antrum of OLGA 0-II stages grouped in Hp-positive and Hp-negative individuals. (A) Semi-quantitative scoring (IQ) of survivin staining in antrum glands in Hp-positive and Hp-negative individuals; n=23, *p=0.0279; mean ± SEM; (B) IQ score of survivin staining in corpus glands in Hp-positive and Hp-negative individuals; n=22, *p=0.0412, mean ± SEM; (C) Representative survivin immunostaining in antrum and corpus gastric tissue. Staining is visualised as a brown pigment. All samples were counterstained with haematoxylin (blue: nuclei). Scale bar: 50 mm.
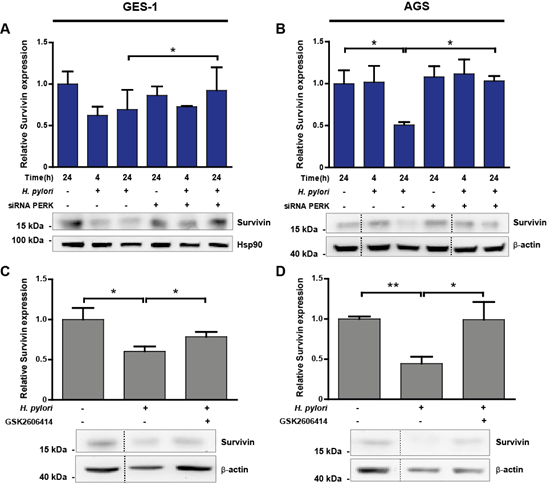
Figure 5: (A-B) Representative immunoblots with the corresponding graphic quantifications showing the mean relative protein abundance of survivin (normalised to b-actin or Hsp90 abundance) in uninfected (H. pylori -) and Hp-infected (H. pylori +) GES-1 (A) and AGS (B) cells. Gastric cells were infected with Hp for 4 and 24 h; in parallel, uninfected control cells were kept in medium for 24 h. PERK silencing (siRNA PERK) was achieved by transfecting GES-1 or AGS cells with 100 nM PERK siRNA 16 h before Hp infection. Non-specific siRNA (100 nM) was used as control (-). (A) n=3, *p<0.05; mean + SEM; (B) n=3, *p<0.05; mean + SEM. (C-D) Survivin protein abundance in uninfected (H. pylori -) and Hp-infected (H. pylori +) GES-1 (C) and AGS (D) cells after 24 h. Representative immunoblots with the corresponding graphic quantifications showing the mean relative protein abundance of survivin (normalised to b-actin abundance). GES-1 and AGS cells were pre-incubated with 25 mM GSK2606414, a selective PERK inhibitor, 1 h before Hp infection. (C) n=4, *p<0.05; mean + SEM; (D) n=3, **p<0.01, * p<0.05; mean + SEM. Dotted lines indicate where lanes, from the same immunoblot, have been removed for clarity.
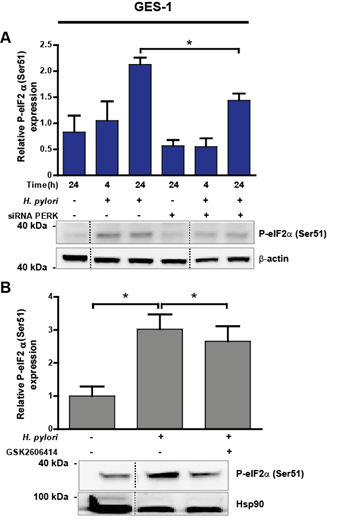
Figure 6: (A) eIF2a protein activation, measured as P-eIF2a(Ser51), in uninfected (H. pylori -) and Hp-treated (H. pylori +) GES-1 cells after 4 and 24 h. Representative immunoblots with corresponding graphic quantification showing mean relative protein abundance of P-eIF2a(Ser51) (normalised to b-actin abundance). Gastric cells were infected with Hp for 4 and 24 h; in parallel, uninfected control cells were kept in medium for 24 h. PERK silencing (siRNA PERK) was achieved by transfecting GES-1 cells with 100 nM PERK siRNA 16 h before Hp infection. Non-specific siRNA (100 nM) was used as a control (-). n=3, *p<0.05; mean + SEM. (B) P-eIF2a(Ser51) abundance in uninfected (H. pylori -) and Hp-treated (H. pylori +) GES-1 cells after 24 h. Representative immunoblots with the corresponding graphic quantification showing mean relative protein abundance of P-eIF2a(Ser51) (normalised to Hsp90 abundance). GES-1 cells were pre-incubated with 25 mM GSK2606414, a selective PERK inhibitor, 1 h before Hp infection. n=4, *p<0.05; mean + SEM. Dotted lines indicate where lanes, from the same immunoblot, have been removed for clarity.
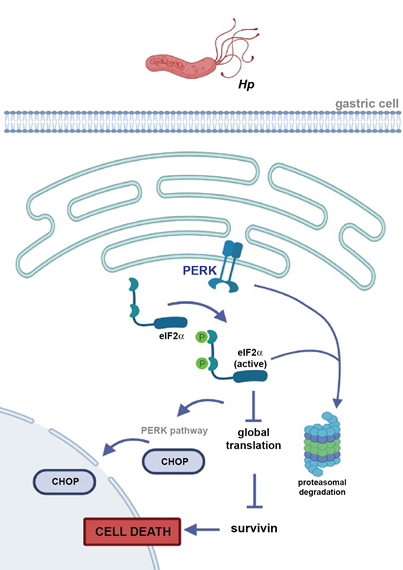
Figure 7: Schematic illustration depicting how PERK activity and/or abundance could contributes to the loss of survivin in gastric cell during Helicobacter pylori (Hp) infection. In non-transformed gastric cells, Hp induces ER stress sensor PERK activation and downstream eIF2a, leading to a reduction in global protein translation and/or increased PERK-dependent proteasomal degradation, which could explain how survivin abundance is reduced. Activation of eIF2a downstream of PERK triggers the canonical apoptosis pathway (via transcription factor C/EBP-homologous protein (CHOP)) resulting in cell death. This sequence of events reported reflects what was observed in tissue samples given that there, Hp infection correlated with increased PERK phosphorylation and loss of survivin. Partly created with BioRender.com.
4. Discussion
Here we determined whether Hp-induced changes in the ER stress sensor PERK contributed to the loss of survivin, previously associated with gastric cell death and the genesis of gastric cancer precancerous lesions. Our results showed that PERK is activated in Hp infection during gastritis in precancerous human gastric mucosa, which correlated with reduced survivin levels compared with Hp-negative gastritis. In vitro, when infected with Hp for 24 h, PERK silencing restored survivin abundance in gastric cell lines, thus mechanistically linking PERK activation to the loss of survivin. Finally, we propose that activation of eIF2a, downstream of the PERK canonical pathway, could represent one mechanism connecting Hp infection to PERK activation, downregulation of survivin and gastric cell death. Consequently, the cellular adaptive ER stress sensor PERK is connected to Hp infection and the reduction in survivin abundance, both in clinical samples and in vitro in gastric cells. Following Hp infection, the loss of survivin via PERK-dependent mechanisms results in gastric cell death, which could favour the genesis and progression of gastric cancer precancerous lesions.
Our data are the first to demonstrate that the UPR sensor, PERK is activated in clinical samples from Hp-positive individuals during OLGA 0-II staged gastritis, suggesting that PERK is chronically activated in Hp-positive compared with Hp-negative gastritis. Total PERK abundance remained unchanged in these samples. These observations agree with our in vitro data, where total PERK abundance was comparable between Hp-infected and non-infected gastric cells. Hp infection induced PERK activation in GES-1 cells, but it decreased in the AGS cell line after 24 h of infection. Instead, discrete activation of PERK was observed at earlier time points following infection. The non-transformed gastric cell line GES-1 used here represents the best available model of a “normal” gastric cell line and the response of these cells to infection likely reflects more closely what happens in the gastric mucosa. As for other gastric cancer cell lines, AGS are a commonly employed gastric adenocarcinoma cell line, with higher basal ER stress levels [43], in which PERK activation upon Hp infection was detected but occurred with different kinetics.
Although PERK activity was markedly increased in GES-1 cells infected by Hp for 24 h, the inhibitory effect of GSK2606414 was partial. GSK2606414 was initially identified as a selective PERK inhibitor [39], by blocking PERK activation in cells and inhibiting tumour growth in human xenograft models in mice [39]. However, for Hp infection of gastric cells the situation appears to be complex. Indeed, GSK2606414 was also demonstrated to inhibit RIPK1, a kinase involved in TNFa-mediated cell death [44] and more recently, KIT, a type III receptor tyrosine kinase, expressed amongst others in the intestinal epithelium [45, 46]. Inhibition of these shared targets by GSK2606414 may lead to compensatory mechanisms that explain the only partial effect of GSK2606414 in Hp-infected gastric GES-1 cells. While interesting, such possibilities remain to be explored in future studies.
So far, other studies have focused on Hp infection, ER stress, UPR activation and gastric cell death [23, 24, 47]. Other UPR components also contribute to cell homeostasis; however, PERK directly contributes to checkpoint function and cell survival through its capacity to regulate cell division [48, 49]. Besides its classical role in the UPR, PERK more recently has been shown to contribute to tumour progression and cell survival [49-51]. However, the role of PERK signalling in tumour development remains controversial [52]. Whereas some studies suggest that PERK activation inhibits tumour cell proliferation leading to apoptosis, in agreement with the classical role of PERK in the UPR [53, 54], others have shown that PERK activation facilitates tumour development by promoting tumour cell survival and enhancing angiogenesis [51, 55, 56]. Consistent with this second role for PERK, there is growing evidence to suggest that PERK is also involved in signalling pathways through UPR-independent functions (PERK non-canonical pathways) [49]. Overall, these observations support the idea that, besides its classical role in UPR and apoptosis, following Hp infection, PERK could also promote pro-survival traits in human gastric cells, such as migration and invasion and thus, the acquisition of a more aggressive phenotype [18].
Here we report that Hp-induced changes in the ER stress sensor PERK contributed to the loss of survivin, an inhibitor of apoptosis, previously associated with gastric cell death and the genesis of gastric cancer precancerous lesions [15, 34]. In addition, our preliminary experiments revealed an increase in the abundance of the pro-apoptotic protein PUMAa/b after exposure to Hp, suggesting that PERK-induced reduced cell viability is specifically linked to decreased survivin abundance and is likely to be independent of other pro-apoptotic BH3-only protein members. Akazawa et al. [23] reported comparable levels of pro-apoptotic BH3-only proteins; however they did so using purified virulence factor vacuolating cytotoxin A on AZ-521 cells. Therefore, we cannot rule out the possibility that the differences with our results relate to the infection of gastric cells with the intact Hp WT26695 strain, which not only expresses vacuolating cytotoxin A, but also other bacterial virulence factors. In contrast to survivin, there was increased abundance of PUMAa/b after 24 h of Hp infection. Thus, the decline in survivin abundance after exposure to Hp points toward a unique mode of regulation involving the ER stress sensor PERK. In agreement, our group has previously reported that the onset of DNA fragmentation and annexin-V/propidium iodide staining were significantly elevated in gastric cells infected with Hp at a MOI 100 for 24 h [34]. Thus, survivin down-regulation upon Hp infection coincides with reduced viability and augmented apoptotic cell death in gastric cells [34].
As yet, both transcriptional regulation [57, 58] and posttranslational degradation via the proteasome [59] have been proposed to play important roles in controlling survivin levels. A previous report from our laboratory showed that, on the one hand, survivin messenger RNA levels are unchanged in the presence of Hp, implying that a posttranscriptional mechanism is responsible for the loss of survivin and, on the other hand, the Hp virulence factor gamma-glutamyl transpeptidase enhanced survivin degradation via the proteasome by a Fe3+-dependent pathway in gastric cells [15]. Our results provide one alternative mechanism, whereby following induction of ER stress and UPR activation, known to occur following Hp infection, both in vivo and in vitro, PERK activity and/or abundance contributes to the loss of survivin (Figure 7). In the non-transformed epithelial gastric cell line GES-1, Hp induces activation of the ER stress sensor PERK, which could partly activate downstream eIF2a, leading to a reduction in global protein translation. This is likely to represent a mechanism by which survivin abundance is reduced. In parallel, eIF2a activation triggers downstream of PERK the canonical apoptosis-related pathway (via CHOP) resulting in cell death (Figure 7). In agreement, an increase in eIF2a activation up to 12 h has been shown to be dependent on the Hp virulence factor vacuolating cytotoxin A in AZ-521 cells [24].
Although Hp-triggered PERK activation in GES-1 cells could lead to increased eIF2a activation and a reduction in survivin abundance, the results from this work also suggest there are additional PERK-dependent mechanisms involved in survivin loss. In accordance, a functional link between eIF2a(Ser51) and proteasomal degradation has been observed [60, 61]. Thus, PERK induces proteasome-dependent degradation via a mechanism requiring eIF2a during ER stress [60]. In addition, PERK activity is a positive regulator of proteasomal activity [62, 63]. This suggests that, in parallel to diminished global protein synthesis due to eIF2a activation, also increased PERK-dependent proteasomal degradation could contribute to survivin down-regulation in GES-1 cells infected by Hp (Figure 7).
5. Conclusion
Our results confirmed that PERK is activated in Hp-positive gastritis and that this correlated with reduced survivin presence as compared to Hp-negative gastritis subject samples. In vitro, we demonstrate that PERK silencing restores survivin abundance following Hp infection, thereby positioning PERK as a key intermediate in the sequence post-infection leading to survivin loss, which could favour the genesis of precancerous gastric lesions. Reduced global protein translation due to PERK and eIF2a activation provide interesting novel targets to prevent survivin protein loss in the gastric mucosa and disease onset.
Author Contributions
Conceptualization, P.D., A.H.C., S.L and A.F.G.Q.; methodology, P.D. and A.Ro.; formal analysis, P.D., A.R. and G. C.; investigation, P.D.; resources, A.H.C., S.L. and A.F.G.Q.; data curation, A.F.G.Q.; writing-original draft preparation, P.D.; writing-review and editing, P.D., A.H.C., S.L. and A.F.G.Q.; visualization, P.D.; supervision, S.L and A.F.G.Q.; project administration, S.L. and A.F.G.Q.; funding acquisition, P.D., A.H.C., S.L. and A.F.G.Q. All authors have read and agree to the published version of the manuscript.
Acknowledgements
This research was funded by Agencia Nacional de Investigación y Desarrollo (ANID) Chile, grants: FONDECYT #3170140 (P.D.), FONDECYT #1170925 (A.F.G.Q.), FONDAP #15130011 (A.F.G.Q., A.H.C., S.L.).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 68 (2018): 394-424.
- Riquelme I, Saavedra K, Espinoza JA, et al. Molecular classification of gastric cancer: Towards a pathway-driven targeted therapy. Oncotarget 6 (2015): 24750-24779.
- Suerbaum S, Michetti P. Helicobacter pylori infection. The New England journal of medicine 347 (2002): 1175-1186.
- Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 153 (2017): 420-429.
- Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta pathologica et microbiologica Scandinavica 64 (1965): 31-49.
- Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet 2 (1975): 58-60.
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer research 52 (1992): 6735-6740.
- Correa P, Piazuelo MB. The gastric precancerous cascade. Journal of digestive diseases 13 (2012): 2-9.
- Sandoval-Borquez A, Saavedra K, Carrasco-Avino G, et al. Noncoding Genomics in Gastric Cancer and the Gastric Precancerous Cascade: Pathogenesis and Biomarkers. Disease markers 2015 (2015): 503762.
- Mera R, Fontham ET, Bravo LE, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut 54 (2005): 1536-1540.
- Massarrat S, Haj-Sheykholeslami A, Mohamadkhani A, et al. Precancerous conditions after H. pylori eradication: a randomized double blind study in first degree relatives of gastric cancer patients. Archives of Iranian medicine 15 (2012): 664-669.
- Chen HN, Wang Z, Li X, et al. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 19 (2016): 166-175.
- Peek RM, Jr., Crabtree JE. Helicobacter infection and gastric neoplasia. The Journal of pathology 208 (2006): 233-248.
- Polk DB, Peek RM, Jr. Helicobacter pylori: gastric cancer and beyond. Nature reviews Cancer 10 (2010): 403-414.
- Valenzuela M, Bravo D, Canales J, et al. Helicobacter pylori-induced loss of survivin and gastric cell viability is attributable to secreted bacterial gamma-glutamyl transpeptidase activity. The Journal of infectious diseases 208 (2013): 1131-1141.
- Bravo J, Diaz P, Corvalan AH, et al. A Novel Role for Helicobacter pylori Gamma-Glutamyltranspeptidase in Regulating Autophagy and Bacterial Internalization in Human Gastric Cells. Cancers 11 (2019).
- Ashktorab H, Dashwood RH, Dashwood MM, et al. H. pylori-induced apoptosis in human gastric cancer cells mediated via the release of apoptosis-inducing factor from mitochondria. Helicobacter 13 (2008): 506-517.
- Diaz P, Valenzuela Valderrama M, Bravo J, et al. Helicobacter pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression. Frontiers in microbiology 9 (2018): 5.
- Peek RM, Jr., Moss SF, Tham KT, et al. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. Journal of the National Cancer Institute 89 (1997): 863-868.
- Suzuki M, Mimuro H, Kiga K, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell host and microbe 5 (2009): 23-34.
- Saadat I, Higashi H, Obuse C, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447 (2007): 330-333.
- Nagy TA, Frey MR, Yan F, et al. Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. The Journal of infectious diseases 199 (2009): 641-651.
- Akazawa Y, Isomoto H, Matsushima K, et al. Endoplasmic reticulum stress contributes to Helicobacter pylori VacA-induced apoptosis. PloS one 8 (2013): e82322.
- Zhu P, Xue J, Zhang ZJ, et al. Helicobacter pylori VacA induces autophagic cell death in gastric epithelial cells via the endoplasmic reticulum stress pathway. Cell death & disease 8 (2017): 3207.
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nature reviews Molecular cell biology 13 (2012): 89-102.
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397 (1999): 271-274.
- Baird TD, Wek RC. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv Nutr 3 (2012): 307-321.
- Puthalakath H, O'Reilly LA, Gunn P, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129 (2007): 1337-1349.
- Ghosh AP, Klocke BJ, Ballestas ME, et al. CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress. PloS one 7 (2012): e39586.
- Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Current opinion in cell biology 17 (2005): 617-625.
- Namba T, Hoshino T, Suemasu S, et al. Suppression of expression of endoplasmic reticulum chaperones by Helicobacter pylori and its role in exacerbation of non-steroidal anti-inflammatory drug-induced gastric lesions. The Journal of biological chemistry 285 (2010): 37302-37313.
- Garg H, Suri P, Gupta JC, et al. Survivin: a unique target for tumor therapy. Cancer cell international 16 (2016): 49.
- Wang TT, Qian XP, Liu BR. Survivin: potential role in diagnosis, prognosis and targeted therapy of gastric cancer. World journal of gastroenterology 13 (2007): 2784-2790.
- Valenzuela M, Perez-Perez G, Corvalan AH, et al. Helicobacter pylori-induced loss of the inhibitor-of-apoptosis protein survivin is linked to gastritis and death of human gastric cells. The Journal of infectious diseases 202 (2010): 1021-1030.
- Rugge M, Pennelli G, Pilozzi E, et al. Gastritis: the histology report. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 43 Suppl 4 (2011): 373-384.
- Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. The American journal of surgical pathology 20 (1996): 1161-1181.
- Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Annals of translational medicine 3 (2015): 9.
- Montgomery EA, Martin DF, Peura DA. Rapid diagnosis of Campylobacter pylori by Gram's stain. American journal of clinical pathology 90 (1988): 606-609.
- Axten JM, Medina JR, Feng Y, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). Journal of medicinal chemistry 55 (2012): 7193-7207.
- Jamison S, Lin Y, Lin W. Pancreatic endoplasmic reticulum kinase activation promotes medulloblastoma cell migration and invasion through induction of vascular endothelial growth factor A. PloS one 10 (2015): e0120252.
- Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene 27 Suppl 1 (2008): 71-83.
- Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America 101 (2004): 11269-11274.
- Wu J, Chen S, Liu H, et al. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. Journal of experimental & clinical cancer research : CR 37 (2018): 272.
- Rojas-Rivera D, Delvaeye T, Roelandt R, et al. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell death and differentiation 24 (2017): 1100-1110.
- Stankov K, Popovic S, Mikov M. C-KIT signaling in cancer treatment. Current pharmaceutical design 20 (2014): 2849-2880.
- Mahameed M, Wilhelm T, Darawshi O, et al. The unfolded protein response modulators GSK2606414 and KIRA6 are potent KIT inhibitors. Cell death & disease 10 (2019): 300.
- Baird M, Woon Ang P, Clark I, et al. The unfolded protein response is activated in Helicobacter-induced gastric carcinogenesis in a non-cell autonomous manner. Laboratory investigation; a journal of technical methods and pathology 93 (2013): 112-122.
- Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proceedings of the National Academy of Sciences of the United States of America 97 (2000): 12625-12630.
- Pytel D, Majsterek I, Diehl JA. Tumor progression and the different faces of the PERK kinase. Oncogene 35 (2016): 1207-1215.
- Rozpedek W, Pytel D, Mucha B, et al. The role of the PERK/eIF2alpha/ATF4/CHOP signaling pathway in tumor progression during Endoplasmic Reticulum stress. Current molecular medicine 16 (2016): 533-544.
- Feng YX, Jin DX, Sokol ES, et al. Cancer-specific PERK signaling drives invasion and metastasis through CREB3L1. Nature communications 8 (2017): 1079.
- Ranganathan AC, Ojha S, Kourtidis A, et al. Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer research 68 (2008): 3260-3268.
- Huber AL, Lebeau J, Guillaumot P, et al. p58(IPK)-mediated attenuation of the proapoptotic PERK-CHOP pathway allows malignant progression upon low glucose. Molecular cell 49 (2013): 1049-1059.
- Martin-Perez R, Palacios C, Yerbes R, et al. Activated ERBB2/HER2 licenses sensitivity to apoptosis upon endoplasmic reticulum stress through a PERK-dependent pathway. Cancer research 74 (2014): 1766-1777.
- Atkins C, Liu Q, Minthorn E, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer research 73 (2013): 1993-2002.
- Mujcic H, Nagelkerke A, Rouschop KM, et al. Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clinical cancer research: an official journal of the American Association for Cancer Research 19 (2013): 6126-6137.
- Torres VA, Tapia JC, Rodriguez DA, et al. Caveolin-1 controls cell proliferation and cell death by suppressing expression of the inhibitor of apoptosis protein survivin. Journal of cell science 119 (2006): 1812-1823.
- Hoffman WH, Biade S, Zilfou JT, et al. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. The Journal of biological chemistry 277 (2002): 3247-3257.
- Zhao J, Tenev T, Martins LM, et al. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. Journal of cell science 113 Pt 23 (2000): 4363-4371.
- Raven JF, Baltzis D, Wang S, et al. PKR and PKR-like endoplasmic reticulum kinase induce the proteasome-dependent degradation of cyclin D1 via a mechanism requiring eukaryotic initiation factor 2alpha phosphorylation. The Journal of biological chemistry 283 (2008): 3097-3108.
- Higgins R, Gendron JM, Rising L, et al. The Unfolded Protein Response Triggers Site-Specific Regulatory Ubiquitylation of 40S Ribosomal Proteins. Molecular cell 59 (2015): 35-49.
- Baltzis D, Pluquet O, Papadakis AI, et al. The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. The Journal of biological chemistry 282 (2007): 31675-31687.
- Gupta S, McGrath B, Cavener DR. PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes 59 (2010): 1937-1947.
