Formic Acid Generation from Impregnated Cellulose with Iron Nanoparticles via Microwave-Assisted Processes
Article Information
Bassem Kamal Abdelkader, Richard Ahorsu, Magda Constanti, Francesc Medina*
Departament d’Enginyeria Química, Universität, Rovira i Virgili, 43007, Tarragona, Spain
*Corresponding Author: Francesc Medina, Departament d’Enginyeria Química, Universität, Rovira i Virgili, 43007, Tarragona, Spain
Received: 24 September 2021; Accepted: 15 November 2021; Published: 27 December 2021
Citation:
Bassem Kamal Abdelkader, Richard Ahorsu, Magda Constanti, Francesc Medina. Selective Formic Acid Generation from Impregnated Cellulose with Iron Nanoparticles via Microwave-Assisted Processes. Journal of Nanotechnology Research 3 (2021): 071-081.
View / Download Pdf Share at FacebookAbstract
The enormous potential of formic acid (FA) as hydrogen carrier, high hydrogen content and low toxicity makes it a good alternative for hydrogen production. Direct generation of iron nanoparticles loosely incorporated in cellulose offered simple route to form FA. The presence of charged ions from alkali salt assisted in the deconstruction of cellulose to glucose and subsequent transformation of glucose to FA concentration of 0.47gC/L. Formic acid selectivity reached 15.8% at a reaction temperature of 180 °C. Employing iron nanoparticles and microwave process in FA production is an environmentally sustainable route. The above processes pave the way for lignocellulosic biomass usage for FA production and making economical usage of catalysts.
Keywords
Formic acid; Microwave; Biomass; Iron oxide
Formic acid articles Formic acid Research articles Formic acid review articles Formic acid PubMed articles Formic acid PubMed Central articles Formic acid 2023 articles Formic acid 2024 articles Formic acid Scopus articles Formic acid impact factor journals Formic acid Scopus journals Formic acid PubMed journals Formic acid medical journals Formic acid free journals Formic acid best journals Formic acid top journals Formic acid free medical journals Formic acid famous journals Formic acid Google Scholar indexed journals Microwave articles Microwave Research articles Microwave review articles Microwave PubMed articles Microwave PubMed Central articles Microwave 2023 articles Microwave 2024 articles Microwave Scopus articles Microwave impact factor journals Microwave Scopus journals Microwave PubMed journals Microwave medical journals Microwave free journals Microwave best journals Microwave top journals Microwave free medical journals Microwave famous journals Microwave Google Scholar indexed journals Biomass articles Biomass Research articles Biomass review articles Biomass PubMed articles Biomass PubMed Central articles Biomass 2023 articles Biomass 2024 articles Biomass Scopus articles Biomass impact factor journals Biomass Scopus journals Biomass PubMed journals Biomass medical journals Biomass free journals Biomass best journals Biomass top journals Biomass free medical journals Biomass famous journals Biomass Google Scholar indexed journals "Iron oxide articles Iron oxide Research articles Iron oxide review articles Iron oxide PubMed articles Iron oxide PubMed Central articles Iron oxide 2023 articles Iron oxide 2024 articles Iron oxide Scopus articles Iron oxide impact factor journals Iron oxide Scopus journals Iron oxide PubMed journals Iron oxide medical journals Iron oxide free journals Iron oxide best journals Iron oxide top journals Iron oxide free medical journals Iron oxide famous journals Iron oxide Google Scholar indexed journals " organic articles organic Research articles organic review articles organic PubMed articles organic PubMed Central articles organic 2023 articles organic 2024 articles organic Scopus articles organic impact factor journals organic Scopus journals organic PubMed journals organic medical journals organic free journals organic best journals organic top journals organic free medical journals organic famous journals organic Google Scholar indexed journals biomass articles biomass Research articles biomass review articles biomass PubMed articles biomass PubMed Central articles biomass 2023 articles biomass 2024 articles biomass Scopus articles biomass impact factor journals biomass Scopus journals biomass PubMed journals biomass medical journals biomass free journals biomass best journals biomass top journals biomass free medical journals biomass famous journals biomass Google Scholar indexed journals hydrogen articles hydrogen Research articles hydrogen review articles hydrogen PubMed articles hydrogen PubMed Central articles hydrogen 2023 articles hydrogen 2024 articles hydrogen Scopus articles hydrogen impact factor journals hydrogen Scopus journals hydrogen PubMed journals hydrogen medical journals hydrogen free journals hydrogen best journals hydrogen top journals hydrogen free medical journals hydrogen famous journals hydrogen Google Scholar indexed journals non-toxic articles non-toxic Research articles non-toxic review articles non-toxic PubMed articles non-toxic PubMed Central articles non-toxic 2023 articles non-toxic 2024 articles non-toxic Scopus articles non-toxic impact factor journals non-toxic Scopus journals non-toxic PubMed journals non-toxic medical journals non-toxic free journals non-toxic best journals non-toxic top journals non-toxic free medical journals non-toxic famous journals non-toxic Google Scholar indexed journals dehydrogenation articles dehydrogenation Research articles dehydrogenation review articles dehydrogenation PubMed articles dehydrogenation PubMed Central articles dehydrogenation 2023 articles dehydrogenation 2024 articles dehydrogenation Scopus articles dehydrogenation impact factor journals dehydrogenation Scopus journals dehydrogenation PubMed journals dehydrogenation medical journals dehydrogenation free journals dehydrogenation best journals dehydrogenation top journals dehydrogenation free medical journals dehydrogenation famous journals dehydrogenation Google Scholar indexed journals biomass articles biomass Research articles biomass review articles biomass PubMed articles biomass PubMed Central articles biomass 2023 articles biomass 2024 articles biomass Scopus articles biomass impact factor journals biomass Scopus journals biomass PubMed journals biomass medical journals biomass free journals biomass best journals biomass top journals biomass free medical journals biomass famous journals biomass Google Scholar indexed journals
Article Details
1. Introduction
Formic acid (FA) is the simplest yet strongest organic acid with significant advantages. It is a commonly used as reductant, a green solvent, and a building block in a variety of chemical syntheses. The overall annual global market for FA is approximately US$ 620 million in 2019 and is expected to continue growing at a consistent rate in the future [1]. Formic acid (FA), which is one of the significant products formed from deconstruction of cellulosic component of biomass, has been considered a propitious liquid hydrogen carrier.
FA is non-toxic, highly stable, and has great hydrogen capacity (4.4 wt %) [2] which is close to the US Department of Energy's target of 5.5 wt% for efficient H2 storage compounds [3]. Hydrogen stored in FA can be released in situ on demand by catalytic dehydrogenation (HCOOH → H2 +CO2). Even though CO2 is released as by-product, it does not contribute to global warming because it can be converted into biomass again through photosynthesis at a shorter life span, as argued by Shimura and Yoshida (2011) [4].
Lignocellulosic biomass is regarded as a viable and sustainable feedstock [5]. FA generation from biomass in the liquid phase requires an appropriate pretreatment step due to the recalcitrance of biomass. Various pretreatment methods such as milling, acid pretreatment, steam explosion, ionic liquids, and Ammonia Fiber Explosion (AFEX) offer promising advantages. However, their disadvantages remain a bottleneck in the deconstruction of biomass. The high energy requirement and capital cost of equipment are the most significant shortcomings of milling pretreatment [6].
For acid pretreatment, usually mineral acids in both concentrated or diluted form are used [7]. Concentrated acid hydrolysis has the advantage of reducing energy consumption as it can be used at atmospheric temperature and pressure. However, it is corrosive to equipment [8]. Using dilute acid requires a reaction to being performed at high temperatures. Due to this, the biomass under deconstruction does first undergo reaction at a mild condition to hydrolyze hemicellulose component then followed by reaction of dilute acid at high temperature. The two-step process could contribute to the overall production cost. In view with this homogeneous catalyst, acid recovery and neutralization have always been a bottleneck. To solve the above problem, various solid acid catalysts have been under study to hydrolyze cellulose to release glucose, which is subsequently transformed into FA. Supported solid acid/metal oxides catalysts are known to have large surface acid species, which much desired for cellulose deconstruction. Solid acids function the same as H+ for cellulose hydrolysis, sulfonated metal oxides can give many acidic species [9]. Such solid acids are mostly synthesized by impregnating the hydroxides from ammonia precipitation of corresponding metal salt solutions with aqueous sulfuric acids followed by calcination (Fukuoka and Dhepe, 2006) [10]. The most popular method for producing FA from cellulose with both high yields and good purity is vanadium(V)-catalyzed oxidation with O2 in acidic aqueous environments. Vanadium-based catalysts, on the other hand, are difficult to make and expensive when compared to iron-based catalysts.
In this study, we synthesized various solid acid /metal oxide and solid acid/ metal hydroxide catalysts by directly impregnating cellulose with Iron (III) nitrate nonahydrate and sulfuric acid. Prior to impregnation of acidic cellulose component, the catalyst was synthesized with only Iron (III) nitrate nonahydrate in the presence of salt (NaNO3). The hydrolysis reaction was driven by microwave processes. The microwave processes serve two purposes (i) to reduce reaction time hence saving energy and (ii) in situ generation of Iron oxide nanoparticles. The effect of NaNO3 and the presence of sulfonate on FA generation were further investigated.
2. Materials and Methods
2.1 Materials
Commercial microcrystalline cellulose powder (Sigma Aldrich), Iron (III) nitrate nonahydrate (Sigma Aldrich 98%), Sulfuric acid (Sigma Aldrich > 95 %, SG =1.83), Sodium hydroxide pellets (Sigma Aldrich 98%), glucose (99%, Panreac), levulinic acid (Sigma Aldrich 98%), formic acid (Fluka 98%), hydroxymethylfurfural (HMF) (Sigma Aldrich 99%).
2.1.1 Material synthesis: Five materials were prepared and denoted as A, B, C, DS and ES. These materials were labeled after every reaction at a specific temperature. That is, reaction at 120 °C; A120, B120, C120, DS120 and ES120, At
140 °C; A140, B140, C140, DS140 and ES140, At
160 °C: A160, B160, C160, DS160 and ES160. At
180 °C; A180, B180, C180, DS180 and ES180.
2.1.2 Synthesis of Material (A): Material A is cellulose impregnated with 3% w/w sulfuric acid. An amount of 10 g of cellulose was impregnated with 15 ml of 0.204 M sulfuric acid solution and dried overnight at 95° C. It was used as the control for experiments.
2.1.3 Synthesis of material (B): Material B contains 3% w/w iron and significant sodium salt (sodium nitrate) content. An amount of 10 g of cellulose was impregnated with 15 ml of 0.358 M solution of iron salt Fe (NO3)3. 9H2O, dried overnight at 95 °C.
Then, the material was immersed in 500 ml of 7M NaOH solution, filtered, washed with 1250 ml washing solution (acetone: water) (60%:40%) v/v respectively, then filtered again. Followed by vacuum drying for 30 min and further dried in oven overnight at 95 °C.
2.1.4 Synthesis of material (C): Material C contains iron 3% w/w of iron and small content of sodium salt. An amount of 5g cellulose impregnated with 3% iron as explained above was gradually added to 200ml NaOH solution of pH=12 till the pH became 10, filtered, washed twice each time with 1000 ml of washing solution (acetone: water) (60%:40%) v/v respectively, filtered again, then vacuum dried for 30 min overnight before drying in oven overnight at 95 °C.
2.1.5 Synthesis of materials (DS) and (ES) from material B and C: Materials DS and ES were prepared from materials B and C respectively to check the effect of adding sulfate species. They were prepared before each reaction by impregnation of 0.5 g of sample with 0.75 ml of 0.204 M sulfuric acid solution. Then dried in oven overnight at 95 °C. See scheme 1.
Scheme 1: Overview of material preparation and hydrolysis.
2.1.6 Characterization of materials: The content of iron and sodium for materials B and C was determined by (ICP-OES, Spectro Arcos, Model:160CCD). An amount of 100 mg of each sample was completely dissolved in concentrated sulfuric acid then diluted with milli-Q water so that the acid concentration in the diluted solution is not more than 2% v/v.
ESEM (environmental scanning electron microscopy) Quanta 600 was used for microscopy study in backscattered configuration with an operational voltage of 20kV. The concentration of total organic carbon in the liquid hydrolysates was determined by total organic carbon (TOC) analyzer (Shimadzu, TOC-L CSN). A volume of 1ml of each liquid sample was diluted in 250 ml solution, then analyzed with the TOC analyzer.
The hydrolysis products were quantified using HPLC (HPLC Agilent tech, 1100 series) with ICSep ICE-COREGEL 87H3 (Column serial nº12525124) as column, using diode array detector (DAD) at 210 nm and refractive index detector (RID). The column operated at 50 °C. The mobile phase was deionized water with a concentration of 0.005M H2SO4. The flow rate of mobile phase was 0.6 mL/min. Sample injection volume was 20 µL. Peak detection time was 50 min.
Standards of glucose, levulinic acid, formic acid and HMF were used for the analysis.
The conversion of cellulose was calculated from the following equation (Eq.1):
Where TOC is the total organic carbon of the liquid hydrolysates, and cellulose is the sample loaded in the reactor. The FA selectivity was calculated from the following equation (Eq.2):
Where FA concentration is the value determined with HPLC expressed in gC/L.
2.1.7 Hydrolysis experiments: Several hydrolysis experiments were carried out at different temperatures with all materials at 120 °C, 140 °C, 160 °C, and 180 °C for 2h in glass vials with modified Polytetrafluoroethylene (TFM) covers with loading 0.5 g sample in 20 ml milli Q water. The reactors were inside Polytetrafluoroethylene (PTFE) vessel filled with 350 ml milli Q water. Samples were irradiated using microwave system (Milestone synthWAVE) with 1200W power and stirring rate of 60%. After hydrolysis, samples were centrifuged, and liquid hydrolysates were analyzed with TOC and HPLC. Solids were washed with 20 ml of washing solution (acetone: water) (60%:40 %) v/v, filtered and dried overnight at 95 °C. Each experiment was carried out in duplicates.
3. Results
Inductively Coupled Plasma Optical Emission Spectrometry analysis revealed the composition of elemental constituents in material B and C see Table 1. The quantitative representation of Fe and Na found in material B and C is evident to the catalytic activity of the as-synthesized material. Moreover, the expected quantitative variation of Fe and Na were also revealed. Figure 1a, ESEM image of the as- synthesized material appeared to have a smooth surface with a probable iron hydroxide imbedded inside the material. EDS/ESEM elemental mapping also revealed Fe and Na in the as-synthesized material of Figure 1a (not shown). Qualitatively, ESEM images gave a mixed particles of iron oxides and sodium which are confirmed by EDS analysis, see Figures 1b and 1c respectively.
|
Element content |
Material B |
Material C |
|
Fe |
2.02% |
3.82 % |
|
Na |
7.37 % |
0.5147 % |
Table 1: Content of elements in samples expressed in (mg element/mg sample) %.
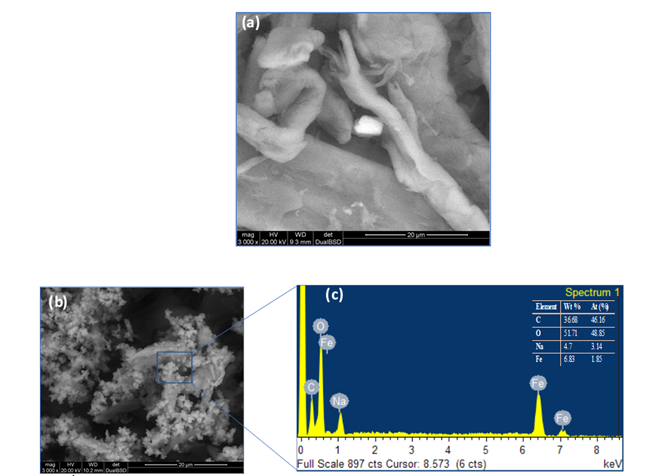
Figure 1: ESEM image of synthesized material; before microwave irradiation (a) after microwave irradiation at 180 °C (b) EDS spectrum (c).
3.1 Effects of salt and Fe on cellulose conversion
As synthesized material containing Fe was not visible until the material was irradiated at 180 °C as revealed by ESEM images see Figures 1(a), 1(b). The visible iron oxide nanoparticles were further confirmed by EDS analysis as seen in the spectrum in Figure 1(c), with presence of elemental Fe, O, Na, and C. Figure 1(a) shows that the conversion of cellulose was highest for material B120 (15.3 %) and lowest for material C120 at 120 °C (10.43%), also materials DS120, ES120 showed conversion with values 14.67% and 14.23% respectively, and that is higher than that of material A120 with a conversion of 13.06%. It can be asserted that as-prepared materials (A, B, C, D and E) showed different conversion rates at a specific temperature. However, the presence of sulfuric acid in B120, C120, DS120 and ES120 did not enhance the conversion of cellulose as was expected. A comparison between materials B120, C120 showed that the high sodium salt content in the case of material B120 gives higher conversion compared to the low salt content in C120. Potvin et al. found the effects of NaCl on the selective conversion of cellulose to levulinic acid [11]. In this work, we observed that iron hydroxide and sodium nitrate assisted with microwave reaction depolymerize cellulose into a formic acid pathway. It has been hypothesized that a higher concentration of ions allows for a significant form of charged particles interacting with the hydrogen bonding network of cellulose at elevated temperature [11].
However, we observed this salt effect at a lower temperature, 120 °C, where high amount of Na salt in sample in B120 enhanced cellulose conversion compared to cellulose conversion observed for C120, Figure 2(a). In Figure 1(b), hydrolysis at 140 °C for 2 h showed a conversion of 21.3%, 17.4% for B140 and DS140, respectively. A140 gave a conversion of 15.3%, that was lower than B140 and DS140. However, A140 hydrolysis showed glucose as the major product without further transformation into formic acid. Material A has no iron content; hence no FA formation was observed. The conversion of material C140 and ES140 was 8.9% and 8.3%, respectively. This low conversion is due to the small content of salt present in C140 and probably in ES140 (prepared from sample C, salt amount not shown) samples, see Table 1 for Na salt amount in C material. It was observed that, the addition of 3% sulfuric acid did not assist in the enhancement of cellulose conversion. This observation was consistent with material B120, C120, DS120, ES120, B140, C140, DS140, and ES140 see Figure (1a) and (1b). Figure 1(a) and (1b) have similar profile as in Figure 1(c), that is a reaction at 160 °C.
However, when the temperature was increased to 180 °C, the conversion trend of cellulose changed, see Figure 1 (d). The conversion of cellulose was doubled for A180 compared to A160, and the selectivity towards glucose was highest. D180 showed higher conversion than B180 with values 29.9% and 26.6 %, respectively.
The lowest conversions recorded for samples C180 and ES180 were 14.9 % and 15.5 %, respectively, at a reaction temperature of 180 °C. It was well evident that elevated temperatures had a significant impact on cellulose conversion with minimum catalytic impact from NaNO3 and H2SO4.
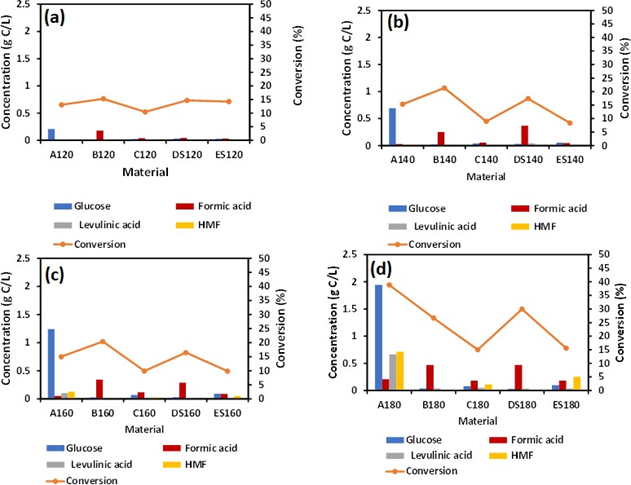
Figure 2: Hydrolysis of as synthesized material at (a) 120 °C (b) 140 °C(c) 160 °C (d) 180 °C for 2h.
3.2 Effects of temperature on formic acid production
From Figure 2(a), no effect of temperature on various as-synthesized material B120, C120, DS120, ES120 was observed within reaction temperature 120 °C. The concentration of glucose 0.2 gC/L recorded for A180 (control) was highest compared to other as- synthesized materials. This suggests that at a lower temperature, the catalytic activity of iron hydroxide and the salt effect was not dependent on temperature. However, FA concentration was lower in material A (A120) compared to other as-synthesized materials. We hypothesized that, at a lower temperature (120 °C), the first stage of cellulose conversion to glucose was driven by the presence of sulfuric acid rather than temperature. The concentration of levulinic acid and HMF was also lower in A120 compared to as-synthesized B120, C120, DS120, and ES120. When the reaction temperature was increased to 140 °C, the glucose concentration also increased to 0.79 gC/L with lower concentrations of levulinic acid and HMF, see Figure 1(b). For as- synthesized B140, C140, DS140, and ES140, the concentration of levulinic acid and HMF were higher. This phenomenon was seen when the temperature was increased to 160 °C. Reaction at elevated temperature of 180 °C, revealed the highest concentration of glucose, levulinic acid, and HMF with 1.9 gC/L, 0.66 gC/L, 0.7 g/L, respectively. This increase in glucose concentration was due to the fact that the thermal transformation of amorphous cellulose took place between 180 °C and 230 °C [12]. Again, the higher temperature did not directly enhance glucose transformation into FA rather than the catalytic activity of Fe nanoparticles. Previous literature shows that, Na salts are selective towards levulinic acid formation [11] while Fe nanoparticles favored FA generation [13]. In this work the catalytic activity Fe was more competitive in directing FA production route.
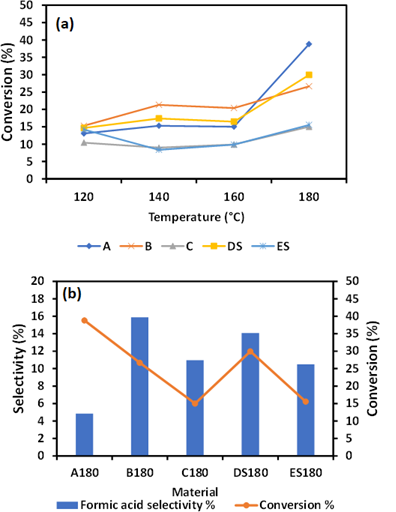
Figure 3: Cellulose conversion as a function of temperature (a) selectivity of formic acid after hydrolysis at 180 °C for 2h (b).
3.3 Selectivity of Formic acid
As shown in Figure 3(a), the reaction temperature has a greater effect on the conversion of cellulose but not an enhancement of formic acid. This implies that metal ions/iron oxides can alter product selectivity but cannot stimulate the degradation of cellulose in a substantial way. The selectivity of formic acid was highest in B180 than any other as-synthesized material A180, C180, D1S80, and ES180. The concentration of formic acid recorded in various as synthesized materials was 0.2 gC/L, 0.47gC/L, 0.18 gC/L, 0.46 gC/L 0.18 gC/L. B180 showed a high concentration of formic acid with a corresponding greater selectivity. B180, which has more salt than C180 see table1, have much effect on the transformation of cellulose into formic acid. It has also been indicated that the addition of 3wt% H2SO4 did not enhance the formic acid selectivity. The FA selectivity of 15.8 % recorded by B180 during hydrolysis at 180 for 2h was due to the presence Fe nanoparticles.
Conclusion
In this study, cellulose was impregnated with iron oxide nanoparticles, NaNO3 and H2SO4. The effect of reaction parameters was investigated, as was the competition between sulfuric acid and sodium nitrate. It was revealed that alkali salt also contributed to the deconstruction of cellulose. For FA formation, iron oxide was used as a widely available catalyst.
Acknowledgement
Bassem Kamal Abdelkader is grateful to Rovira I Virgili laboratory support grant. Richard Ahorsu is grateful for the support from Marti Franques grant number 2017PMF-PIPF-43. This work was supported by the Spanish Ministry of Science, Innovation and Universities, project RTI2018- 098310-B-I00 and Diputació de Tarragona.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Chen X, Liu Y, Wu J. Sustainable production of formic acid from biomass and carbon dioxide. Molecular Catalysis 483 (2020): 110716.
- Yadav M, Xu Q. Liquid-phase chemical hydrogen storage materials. Energy and Environmental Science 5 (2012): 9698-9725.
- Sordakis K, Tang C, Vogt LK, et al. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chemical Reviews 118 (2018): 372-433.
- Shimura K, Yoshida H. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy and Environmental Science 4 (2011): 2467-2481.
- Ahorsu R, Cintorrino G, Medina F, et al. Microwave processes: A viable technology for obtaining xylose from walnut shell to produce lactic acid by Bacillus coagulans. Journal of Cleaner Production 231 (2019): 1171-1181.
- Gu BJ, Wang J, Wolcott MP, et al. Increased sugar yield from pre-milled Douglas-fir forest residuals with lower energy consumption by using planetary ball milling. Bioresource Technology 251 (2018): 93-98.
- Gavilà L, Constantí M, Medina F. d-Lactic acid production from cellulose: dilute acid treatment of cellulose assisted by microwave followed by microbial fermentation. Cellulose 22 (2015): 3089-3098.
- Guo F, Fang Z, Xu CC, et al. Solid acid mediated hydrolysis of biomass for producing biofuels. Progress in Energy and Combustion Science 38 (2012): 672-690.
- Ferris KF. Sulfonated Metal-Oxide Surfaces: What Makes Them So Acidic? Symposium Ca – Interface Control of Electrical, Chemical, and Mechanical Properties. MRS Online Proceedings Library (OPL) 318 (1994): 539.
- Fukuoka A, Dhepe PL. Catalytic conversion of cellulose into sugar alcohols. Angewandte Chemie - International Edition 45 (2006): 5161-5163.
- Potvin J, Sorlien E, Hegner J, et al. Effect of NaCl on the conversion of cellulose to glucose and levulinic acid via solid supported acid catalysis. Tetrahedron Letters 52 (2011): 5891-5893.
- Fan J, De Bruyn M, Budarin VL, et al. Direct microwave-assisted hydrothermal depolymerization of cellulose. Journal of the American Chemical Society 135 (2013): 12728-12731.
- Hou Y, Lin Z, Niu M, et al. Conversion of Cellulose into Formic Acid by Iron(III)-Catalyzed Oxidation with O2 in Acidic Aqueous Solutions. ACS Omega 3 (2018): 14910-14917.
