Effects of Testosterone Replacement in Hypogonadal Heart Transplanted Men
Article Information
Doris Wagner1, Karin Amrein2, Julia Mader2, Oliver Malle2, Astrid Fahrleitner-Pammer2*
1Department of Surgery, Medical University of Graz, Austria
2Department of Internal Medicine, Division of Endocrinology and Diabetes, Medical University of Graz, Austria
*Corresponding Author: Dr. Astrid Fahrleitner, Department of Internal Medicine, Division of Endocrinology and Metabolism, Medical University of Graz, Austria
Received: 03 August 2020; Accepted: 20 August 2020; Published: 28 August 2020
Citation: Doris Wagner, Karin Amrein, Julia Mader, Astrid Fahrleitner-Pammer. Effects of Testosterone Replacement in Hypogonadal Heart Transplanted Men. Archives of Nephrology and Urology 3 (2020): 061-076.
View / Download Pdf Share at FacebookAbstract
Context: Hypogonadism is common in cardiac transplant patients and exerts negative effects on bone, but also libido and quality of life.
Objective: We investigated whether testosterone replacement therapy (TRT) on top of ibandronate in hypogonadal CTX recipients is beneficial for important clinical outcomes.
Design: Observational study (eugonadal and hypogonadal) and non-blinded randomized controlled trial (hypogonadal subgroup).
Setting: Academic tertiary care center at the Medical University of Graz, Austria
Patients: 52 heart transplanted men (21 eugonadal, 31 hypogonadal) with untreated osteoporosis (median 50 months post transplantation). 8 patients could not enter the study because of pathological urologic examination.
Intervention(s): Besides standard therapy with ibandronate 2 mg quarterly iv and oral daily calcium/vitamin D, hypogonadal men were randomly assigned to either testosterone replacement (n=14) or not (n=17).
Main Outcome Measures: Bone mineral density (BMD), fracture incidence, sexual function
Results: At baseline, hypogonadal compared to eugonadal men had lower Z-score values at the femoral neck (-1.54 vs. 0.15) and total hip (-1.34 vs. 0.01) (all P<0.001) and significantly more prevalent vertebral fractures (63% vs. 14%, P<0.001). After 5 years, BMD increased in all patients, however, hypogonadal patients with additional TRT showed a higher increase and fracture incidence was significantly lower in patients receiving additional TRT (21% compared to 41%; p= 0.001). Patients on TRT also reported an increase in sexual activities after 1 (29 ± 8; p<0.001) and 5 years (25 ± 9; p<0.001), while no changes were reported by the other groups.
Keywords
Transplantation bone disease; Hypogonadism; Fracture; Testosterone replacement; Immunosuppressive
Transplantation bone disease articles, Hypogonadism articles, Fracture articles, Testosterone replacement articles, Immunosuppressive articles
Transplantation bone disease articles Transplantation bone disease Research articles Transplantation bone disease review articles Transplantation bone disease PubMed articles Transplantation bone disease PubMed Central articles Transplantation bone disease 2023 articles Transplantation bone disease 2024 articles Transplantation bone disease Scopus articles Transplantation bone disease impact factor journals Transplantation bone disease Scopus journals Transplantation bone disease PubMed journals Transplantation bone disease medical journals Transplantation bone disease free journals Transplantation bone disease best journals Transplantation bone disease top journals Transplantation bone disease free medical journals Transplantation bone disease famous journals Transplantation bone disease Google Scholar indexed journals Hypogonadism articles Hypogonadism Research articles Hypogonadism review articles Hypogonadism PubMed articles Hypogonadism PubMed Central articles Hypogonadism 2023 articles Hypogonadism 2024 articles Hypogonadism Scopus articles Hypogonadism impact factor journals Hypogonadism Scopus journals Hypogonadism PubMed journals Hypogonadism medical journals Hypogonadism free journals Hypogonadism best journals Hypogonadism top journals Hypogonadism free medical journals Hypogonadism famous journals Hypogonadism Google Scholar indexed journals Fracture articles Fracture Research articles Fracture review articles Fracture PubMed articles Fracture PubMed Central articles Fracture 2023 articles Fracture 2024 articles Fracture Scopus articles Fracture impact factor journals Fracture Scopus journals Fracture PubMed journals Fracture medical journals Fracture free journals Fracture best journals Fracture top journals Fracture free medical journals Fracture famous journals Fracture Google Scholar indexed journals Testosterone replacement articles Testosterone replacement Research articles Testosterone replacement review articles Testosterone replacement PubMed articles Testosterone replacement PubMed Central articles Testosterone replacement 2023 articles Testosterone replacement 2024 articles Testosterone replacement Scopus articles Testosterone replacement impact factor journals Testosterone replacement Scopus journals Testosterone replacement PubMed journals Testosterone replacement medical journals Testosterone replacement free journals Testosterone replacement best journals Testosterone replacement top journals Testosterone replacement free medical journals Testosterone replacement famous journals Testosterone replacement Google Scholar indexed journals Immunosuppressive articles Immunosuppressive Research articles Immunosuppressive review articles Immunosuppressive PubMed articles Immunosuppressive PubMed Central articles Immunosuppressive 2023 articles Immunosuppressive 2024 articles Immunosuppressive Scopus articles Immunosuppressive impact factor journals Immunosuppressive Scopus journals Immunosuppressive PubMed journals Immunosuppressive medical journals Immunosuppressive free journals Immunosuppressive best journals Immunosuppressive top journals Immunosuppressive free medical journals Immunosuppressive famous journals Immunosuppressive Google Scholar indexed journals Cardiac transplantation articles Cardiac transplantation Research articles Cardiac transplantation review articles Cardiac transplantation PubMed articles Cardiac transplantation PubMed Central articles Cardiac transplantation 2023 articles Cardiac transplantation 2024 articles Cardiac transplantation Scopus articles Cardiac transplantation impact factor journals Cardiac transplantation Scopus journals Cardiac transplantation PubMed journals Cardiac transplantation medical journals Cardiac transplantation free journals Cardiac transplantation best journals Cardiac transplantation top journals Cardiac transplantation free medical journals Cardiac transplantation famous journals Cardiac transplantation Google Scholar indexed journals testosterone replacement therapy articles testosterone replacement therapy Research articles testosterone replacement therapy review articles testosterone replacement therapy PubMed articles testosterone replacement therapy PubMed Central articles testosterone replacement therapy 2023 articles testosterone replacement therapy 2024 articles testosterone replacement therapy Scopus articles testosterone replacement therapy impact factor journals testosterone replacement therapy Scopus journals testosterone replacement therapy PubMed journals testosterone replacement therapy medical journals testosterone replacement therapy free journals testosterone replacement therapy best journals testosterone replacement therapy top journals testosterone replacement therapy free medical journals testosterone replacement therapy famous journals testosterone replacement therapy Google Scholar indexed journals Bone mineral density articles Bone mineral density Research articles Bone mineral density review articles Bone mineral density PubMed articles Bone mineral density PubMed Central articles Bone mineral density 2023 articles Bone mineral density 2024 articles Bone mineral density Scopus articles Bone mineral density impact factor journals Bone mineral density Scopus journals Bone mineral density PubMed journals Bone mineral density medical journals Bone mineral density free journals Bone mineral density best journals Bone mineral density top journals Bone mineral density free medical journals Bone mineral density famous journals Bone mineral density Google Scholar indexed journals
Article Details
1. Introduction
Cardiac transplantation (CTX) is the treatment of choice for patients with terminal heart failure. In the last decades, advances in immunosuppressive therapy have markedly improved short and long term survival [1]. Bone disease causes considerable morbidity and mortality in the pretransplant and the posttransplantation period [2, 3]. Bone loss is exaggerated in the first year following CTX with a decrease that ranges from 3% to 9% at the spine and 6% to 11% at the femoral neck, with individual bone mineral density (BMD) loss of up to 19% [4]. Fracture incidence ranges between 15% and 36% in the first year after CTX [5]. Data on the time after the first year are scarce but it seems that the risk for fractures and bone loss remains high, especially if untreated [6, 7]. The pathogenesis of bone loss after transplantation is complex and mostly triggered by preexisting bone disease aggravated by immunosuppressive therapy [8, 9]. Steroids, cyclosporine and other drugs may lead to renal impairment, secondary hyperparathyroidism and hypogonadism-factors which all exaggerate detrimental effects on bone health [10].
Hypogonadism is an important contributor to male osteoporosis [11]. The prevalence of bone disease is high in hypogonadal men and fracture rates around 10% have been reported for young patients [12]. In studies investigating older men, hip fracture rates around 30% have been reported. Those numbers are similar to women of the same age [13]. Although the important function of androgens for muscles, brain and sexual function is well-known, their specific role for peak bone mass and the maintenance of bone mass throughout adulthood has not been fully investigated [14-16].
Hypogonadism in heart transplanted men has not been investigated widely. Testosterone levels decline in the first month following transplantation but are considered to return to normal again by 6 months after transplantation [17]. Immunsuppressive therapy seems to have a crucial role in the pathogenesis of hypogonadism. Low testosterone levels have been associated with a suppression of the hypothalamic pituitary gonadal axis due to glucocorticoid therapy, a likely explanation for the decrease during the early phase following surgery. However, a direct cytotoxic effect of cyclosporine on the leydig cells has also been described [17], thus both testicular and pituitary function seem to be impaired in this setting.
Although low testosterone levels after CTX have been associated with excessive bone loss, no prospective studies on the effects of testosterone supplementation on bone mass and sexual activities have been published [3, 18]. Interestingly, even in patients without immunosuppressive therapy, data on additional TRT in combination with osteoprotective agents are scarce, but promising. No severe urological adverse effects have been reported [19,20].
Therefore we decided to investigate in an observational study (i) whether or not male hypogonadism is a confounder of transplantation bone disease, and further performed a randomized controlled study to (ii) analyze if osteoprotective therapy with IBN has an effect on bone density independently of gonadal function and (iii) if TRT on top of IBN in hypogonadal patients exerts an additional benefit on bone density, fracture incidence and sexual function.
Patients and Methods
2.1 Study design
This study was designed as an observational study followed by a randomized non-blinded controlled study. It was approved by the ethics committee of the Medical University of Graz. Male patients with osteoporosis who were at least 12 months after CTX and had not received any bone specific medication since transplantation were invited to participate in the study. The study was carried out in the outpatient clinics of the Division of Transplantation and the Division of Endocrinology at the Medical University of Graz. Patients were recruited during their routine follow up control visit. After written informed consent was obtained, serum testosterone and prostate specific antigen (PSA) were determined in all patients, and a urologic assessment was carried out defining patients’ risk for prostatic hyperplasia. Bone health was assessed with dual X ray absorptiometry (DXA), laboratory analysis and standardized X-ray of the spine. Osteoporosis was defined by WHO criteria [21]. Patients with prostate hyperplasia or PSA elevation were excluded. According to their free testosterone level, patients were classified as eugonadal or hypogonadal. The free testosterone (fTest) cutoff level below which patients were classified as hypogonadal was 6.7 pg/mL (normal range 6.7-54.7 pg/mL). Hypogonadal patients were randomized consecutively by a web-based randomization service (www.randomizer.at) to receive either testosterone replacement therapy (TRT group) or not (control group, CTR). The randomization was stratified according to the baseline data of renal function, time since CTX, age, body mass index and Z score values of the total hip.
All three groups received intravenous ibandronate (IBN) 2 mg every three months in combination with oral 1200 mg calcium and 880IU vitamin D daily. This treatment regime using low dose IBN has been proven as an effective therapy in CTX patients with slightly impaired kidney function and triple immunosuppressive therapy [4, 26]. The TRT group received an additional intramuscular injection of 250 mg testosterone enanthate every 4-6 weeks or 50 mg testosterone cutaneous daily. The EU group as well as the control group did not receive testosterone supplementation. A complete study flow chart is given in Figure 1.
The study was planned in 2004 and carried out from 2005 to 2010 with an additional one year follow up. Primary study outcome parameters were differences between eugonadal and hypogonadal patients in BMD, bone markers and fracture prevalence at the time of study entry, as well as treatment response to IBN in dependence of gonadal function. Secondary endpoints included the assessment of additional TRT in hypogonadal patients with respect to changes in BMD, fracture incidence and bone metabolism, sexual activities as well as loss or presence of libido at the different time points assessed between the three groups.
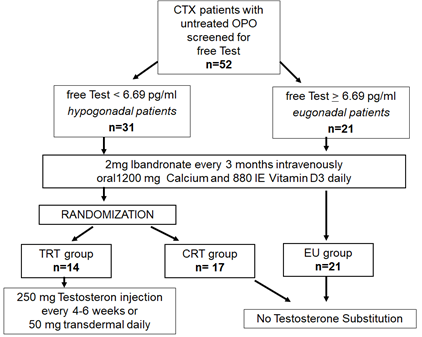
Figure 1: Flowchart.
2.2 Patients
Sixty nine patients with osteoporosis who had received a CTX at our center were screened. 9 patients did not give consent and 8 patients (13%) had to be excluded from the study because of prostatic hyperplasia or suspected cancer identified at urological assessment. Therefore, 52 patients were enrolled. All subjects were advised of the nature of the study and gave written informed consent. No patient died or was lost to follow up during the study period.
2.3 Immunosuppression
All patients received triple immunosuppressive treatment consisting of steroids, cyclosporine A and mycophenolate mofetil. Currently, the average 5-year survival after CTX with this immunosuppressive regimen is 87% [22, 23]. In brief, this regimen consists of methylprednisolone starting with 1 g intravenously at the time of transplantation, 750 mg on the first day after surgery, followed by oral prednisolone started with 15 mg/d for 6 months. If there is no rejection episode, prednisolone is further tapered stepwise by 5 mg every 3 up to 6 months up to a lifelong maintenance dose of 5 mg prednisolone per day. The cyclosporine dose is titrated to target serum cyclosporine levels around 200 ng/mL during the first month, followed by levels around 150 ng/mL during the following 6 months and levels around 100 ng/mL in the time period thereafter. The standard mycophenolate mofetil dosage is 1 g twice daily with dose adaptions and reductions depending on white blood cell count.
2.4 Laboratory analysis
Blood sampling was performed at baseline and every 12 months as part of a routine outpatient visit between 8 and 10 am after an overnight fast. Routine serum parameters were measured by an auto-analyzer (Hitachi 747, Germany); the remaining blood samples were immediately cooled, centrifuged, aliquoted and stored at -70°. Parathyroid hormone (iPTH), osteocalcin (OC) and C-terminal telopeptide of type I collagen (sCTX) were analyzed by electrochemiluminescence immunoassay (“Elecsys 2010”, Roche, Elecsys Systems, Mannheim, Germany).
Bone specific alkaline phosphatase (bALP) and the active isoform 5b of tartrate resistant acid phosphatase (TRAP 5b) were measured by enzyme immunoassay with an intra- and interassay variability of 3.7-6.7% and 7-8.1% respectively (IDS, Boldon, United Kingdom). 25-hydroxyvitamin D [25(OH)D] was assessed by an enzyme immunoassay with an intra- and interassay variability of 5.3-6.7 and 4.6-8.7% (IDS). Total testosterone (tTest) was measured by a competitive chemoluminiscence immunoassay with an intra- and interassay variance of 3.2% and 6.8% (ADVIA Centaur, Siemens Healthcare, Germany). Free testosterone (fTest) was measured by radioimmunoassay (RIA) from DSL (Diagnostic Systems Laboratories Systems, Webster, TX, USA) with a lowest detection limit of 0.18 pg/mL and intra- and interassay variability of 3.7% and 7.3% respectively.
All analyses were performed with kits of the same lot number, with the samples determined in a random order. All samples were measured in duplicate and averaged.
2.5 BMD measurement
Bone mineral density (BMD) was measured by DXA (Lunar iDXA®, GE) at baseline and after 12 as well as after 60 months of treatment. The in vivo coefficient of variation at our institution is 2.1% for the femoral neck, 1.6% for the trochanteric region and 2.3% for the total hip. BMD results are expressed as absolute (g/cm2) and Z-score values (+ SD), which compare individual results to an age-matched database. The BMD at the lumbar spine was not assessed as virtually all patients showed signs of aortic calcification in the X-rays of the spine and a significant percentage (40%, n=21) of the included patients had prevalent vertebral fractures.
2.6 Spine radiographs
Standardized X-rays of the thoracic and lumbar spine were performed at baseline and after 60 months. The anonymized films were analyzed by an experienced radiologist. The severity of vertebral fractures was assessed using semi-quantitative (SQ) visual assessment and these scores were assigned to each individual vertebra from T4 to L4. A reduction of 20% of the anterior, middle or posterior vertebral height was classified as a vertebral fracture [24]. Only a height reduction in a prior normal vertebra was classified as a new vertebral fracture.
2.7 Diagnosis of hypogonadism and assessment of libido
The diagnosis of hypogonadism was performed based on the free testosterone levels that had been measured as described previously. A cutoff level of <6.7 pg/mL was used to define hypogonadism. Signs and symptoms including loss of libido were documented. Additionally, patients filled in the Androgen Deficiency in Aging Male questionnaire (ADAM, Figure 2) that has been proven a strong indicator for hypogonadism with 97% sensitivity [25]. Loss of libido and quality of life were assessed with this questionnaire. Additionally, patients were asked to keep a diary on their sexual events. Sexual intercourses with partners as well as masturbation were recorded as sexual events. The total number was assessed from the patients’ diary and recorded separately.
|
1. The ADAM questionnaire 2. Do you have a decrease in libido or sex drive? 3. Do you have a lack of energy? 4. Do you have a decrease in strength and/or endurance? 5. Have you lost weight? 6. Have you noticed a decreased “enjoyment of life?” 7. Are you sad and/or grumpy? 8. Are your erections less strong? 9. Have you noticed a recent deterioration in your ability to play sports? 10. Are you falling asleep after dinner? 11. Has there been a recent deterioration in your work performance? 12. A positive ADAM questionnaire result was defined as affirmative answers for question 1 and 7 or for any of the other items. Figure 2: The gonadal function of the patients was assessed using the Androgen Deficiency in Aging Male (ADAM) questionnaire. An outline of the used questionnaire is presented. |
2.8 Urologic assessment
All patients who were invited to participate in the presented study underwent urologic examination previous to study entry as described above. During the study period and in the year thereafter, symptoms of prostate hypertrophy were ascertained using the American Urologic Association International Prostate Symptom Score (IPSS, 5 point Lickert scale). This score measures symptoms of incomplete emptying of bladder, frequency of urination, weak urinary system, straining to urinate, nocturia and an evaluation of the effects of urinary symptoms on quality of life; total scores range from 0 to 35. In case of any suspicion, patients were referred for a urologic examination. In addition to the score and the clinical examination, PSA was measured in all subjects every 6 months if the patient received testosterone supplementation and every 12 months if the patient was in the EU or in the CTR group.
2.9 Statistical analysis
All data are presented as median and interquartile range unless otherwise indicated. All data were checked for normality using the Kolmogorov-Smirnov test and logarithmically transformed in case of nonlinearity. For between group comparisons, unpaired Student`s t-test, Mann Whitney U-test or the Chi square test were used accordingly. ANOVA followed by the Fisher´s multiple comparison test was applied to test the differences between groups. Changes in the parameters compared to baseline (BMD, biochemical markers, sexual events etc.were analyzed using the Student`s t-test. All statistical analyses were performed using SPSS 19.0 (IBM, Chicago, IL, USA) and a p < 0.05 was considered significant.
3. Results
3.1 Patient characteristics
In total, 52 men were included in the analysis. The median age was 56 years (interquartile range (IQR): 50;62) with a median of 50 months (20;90) after CTX. The BMI was 26 (24;29) and many patients (33%) had impaired renal function. Most patients had chronic kidney disease (CKD) stage 2 with a glomerular filtration rate (GFR) between 60 and 90 ml/min (38%, n=20) or CKD stage 3 (35%, n=18) with a GFR between 30 and 60 ml/min. Median serum creatinine was 1.7 mg/dL (1.3; 2.1) in the total patient cohort. The median free testosterone (fTest) was 10.9 pg/mL (8.8;16.8). The median 25-hydroxyvitamin D (25OHD) level was 20.4 ng/mL (11;27) with a median intact parathyroid hormone (iPTH) of 45.5 pg/mL (32.3;68.3). The patients received a median prednisolone dose of 7.5 mg daily. Twenty seven percent of the included patients (n=14) had secondary hyperparathyroidism and 48% of the patients had 25OHD levels below 20 ng/mL (n=25). All included patients were screened as described above and divided into three groups: eugonadal patients (EU group, n=21), hypogonadal patients with (TRT group, n=17) or without (CTR group, n=14) testosterone supplementation. Baseline parameters were compared between hypogonadal (TRT group and CTR group: n=31) and eugonadal (EU group; n=21) patients.
The hypogonadal intervention and treatment group were comparable regarding age, time since transplantation, prednisolone dose and biochemical markers (see Tables 1 and 2). Per definition, hypogonadal patients showed significantly lower fTest and tTest levels (p=0.001) when compared to eugonadal patients accompanied by lower Z scores at the total hip (0.01 EU vs. -1.34 hypogonadal patients; p=0.001), the femoral neck (0.51 vs.-1.62, p=0.001) and at the trochanteric region (-0.22 vs.-1.24, p=0.001). Furthermore, fracture prevalence was significantly higher in hypogonadal patients as compared to eugonadal patients. At study entry, the 17 patients in the TRT group had a total number of 10 fractured vertebrae (6 patients had 1, 2 patients had 2) and patients in the CTR group had a total number of 12 fractured vertebrae (6 patients had 1, 3 patients had 2) (Table 1).
|
EU group |
TRT group |
CTR group |
P value TRT vs CTR |
|
|
Numbers of patients |
21 |
14 |
17 |
n.s. |
|
Indications for HTX (%) |
dilated cardiomyopathy 65% (n=14) ischemic cardiomyopathy 35% (n=7) |
dilated cardiomyopathy 83% (n=12) ischemic cardiomyopathy 17% (n=2) |
dilated cardiomyopathy 85% (n=14) ischemic cardiomyopathy 15% (n=3) |
n.s. n.s. |
|
Age (years) |
55 ± 9 |
55 ± 7 |
58 ± 11 |
n.s. |
|
Time since HTX (months) |
32 ± 39 |
58 ± 41 |
63 ± 35 |
n.s. |
|
BMI (kg/m2) |
24 ± 5 |
26 ± 5 |
25 ± 8 |
n.s. |
|
Serum magnesium (0.7-1.1 mmol/L) |
0.83 ± 0.1 |
0.86 ± 0.1 |
0.87 ± 0.2 |
n.s. |
|
Serum calcium (2.2-2.65 mmol/L) |
2.38 ± 0.1 |
2.41 ± 0.1 |
2.45± 0.1 |
n.s. |
|
Parathyroid hormone (15-65 pg/mL) |
51 ± 18 |
44 ± 19 |
46 ± 21 |
n.s. |
|
25OH-hydroxyvitamin D (30-60 ng/mL) |
19 ± 8 |
19 ± 10 |
23 ± 10 |
n.s. |
|
Serum cross laps (0.06-0.35 ng/mL) |
0.36 ± 0.2 |
0.42 ± 0.3 |
0.39 ± 0.2 |
n.s. |
|
Bone alkaline phosphatase (5.7-32.9 ng/mL) |
38 ± 8.3 |
36 ± 13 |
39 ± 13 |
n.s. |
|
Alkaline phosphatase (40-130 ng/mL) |
154 ± 41 |
133 ± 40 |
123 ± 31 |
n.s. |
|
Free testosterone (6.69-54.7 pg/mL) |
12.9 ± 5.3 |
3.8 ± 1.6 |
4.5 ± 2.7 |
n.s. |
|
Total testosterone (2.41-8.3 ng/mL) |
5.6 ± 1 |
3.5 ± 1 |
3.9 ± 1.2 |
n.s. |
|
Vitamin D deficiency (% below 20 ng/ml) |
25 |
64 |
63 |
n.s. |
|
Secondary hyperparathyroidism (%) |
13 |
64 |
65 |
n.s. |
|
Z score total hip (+ SD) |
0.01 ± 0.8 |
-1.51 ± 0.98 |
-1.32 ± 1.05 |
n.s. |
|
Z score femoral neck (+ SD) |
0.47 ± 1.06 |
-1.73 ± 0.91 |
-1.53 ± 1.04 |
n.s. |
|
Z score trochanteric region (+ SD) |
-0.17 ± 0.89 |
-1.51 ± 0.98 |
-1.39 ± 1.15 |
n.s. |
|
Patients with vertebral fractures (%) |
8% (2/21) |
57% (8/14) |
53% (9/17) |
n.s. |
|
Total number of fractures |
2 |
10 |
12 |
n.s. |
|
Prednisolone dosage (mg/day) |
6.7 ± 2 |
6.0 ± 2 |
6.3 ± 2 |
n.s. |
Parameters are expressed as median and standard deviation, to convert Vitamin D levels to nmol/L multiply with 2.496.n.s: not significant
Table 1: Patients characteristics according to group assignment.
3.2 Biochemical markers and changes in BMD
Serum CTX and bALP levels decreased significantly throughout the study in eugonadal patients (month 0 vs. 60: sCTX: 0.39 ± 0.20 vs. 0.22 ± 0.18 ng/mL. p=0.001; bALP: 38 ± 8 vs. 22 ± 7, p<0.001). A significant decrease was also observed in hypogonadal patients without testosterone substitution (sCTX: 0.38 ± 0.16 vs. 0.30 ± 0.06, p=0.03, bALP: 39 ± 13 vs. 22 ± 6, p<0.001) and with testosterone replacement therapy (sCTX: 0.46 ± 0.18 vs. 0.22 ± 0.11, p<0.001; bALP: 37 ± 13 vs. 21 ± 5, p=0.001) (Table 2). The Z-score at all three sites slightly increased in the eugonadal patients and the hypogonadal patients receiving IBN therapy, whereas the TRT patients revealed a significant increase already after one (+25 % ± 4) and five years (23 % ± 3, p<0.001).
*The last row gives the differences of changes of the parameters investigated compared between the hypogonadal patients without hormone replacement therapy with those who received testosterone therapy.
Table 2: The presented table compiles results of the effect of TRT replacement on bone mineral density readings according to the different groups to which the patients were assigned. Patients in the TRT group –i.e. patients who received testosterone supplementation throughout the study period showed a significant decrease of the measured z scores and therefore a significant increase in their BMD.
3.3 Fractures
In the EU group, 3 patients sustained their first vertebral fracture. In the TRT group, only patients with prevalent fractures experienced new fractures-i.e. two patients developed one new fracture and one patient developed three new fractures. In the CTR group, patients with prevalent vertebral fractures developed new fractures (2 patients with 1 and 3 patients with 2 new fractures) but also patients without fractures at study entry sustained fractures despite ongoing bisphosphonate treatment (1 patient with 1 and 1 with 2 fractures) (Table 2). TRT patients had a 74% lower risk to sustain a fresh vertebral fracture throughout the study period as compared to patients without TRT therapy (p=0.001) or eugonadal patients (risk reduction 53%, p=0.03) (Figure 3).
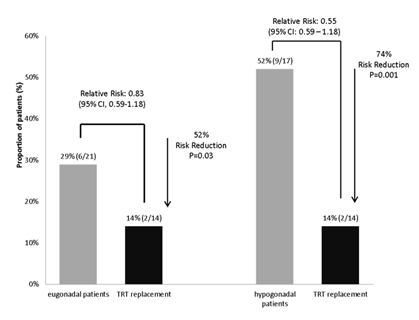
Figure 3: Patients who received TRT had a significantly lower risk to sustain fresh vertrebral fractures as compared to hypogonadal patients and as compared to eugonadal patients. Testosterone replacement was able to reduce the fracture risk by 74% (TRT vs. hypogonadal) and 52% (TRT vs. eugonadal).
3.4 Testosterone levels
fTest and tTest differed significantly between the eugonadal and hypogonadal group at baseline (Table 1). Until the end of the study, tTest in the EU group decreased (p=0.08) by 20% from 5.2 ng/mL at baseline to 4.2 ng/mL at the end of the study. Total testosterone levels in the CTR group remained stable with 3.2 ng/mL at baseline and 3.1 ng/mL at study end. In the TRT group, tTest levels increased significantly (p=0.001) from 3.5 ng/mL at baseline to 6.8 ng/mL at study end as expected. Free testosterone decreased significantly in the EU group throughout the study period and remained stable in the CTR group. As expected, patients in the TRT group showed a significant increase in fTest levels (3.9 ± 1.3 vs. 20.8 ± 13.5, p=0.001) throughout the study period (Figure 4).
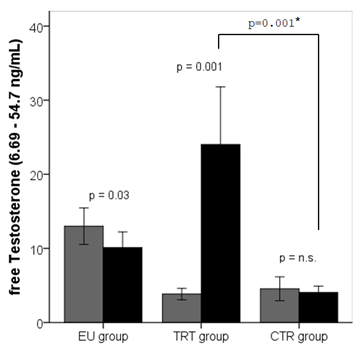
Figure 4: Free testosterone (fTest) levels declined in the EU group (11.7±5.4 vs. 9.1±4.6, p=0.03) throughout the study period and remained stable in the CTR group (4.6±2.7 vs. 4.4±1.6, p=n.s.). Patients in the TRT group showed a significant increase in free testosterone levels (3.9±1.3 vs. 20.8±13.5, p<0.001) throughout the study period. Light grey bars represent the mean levels at baseline and black bars mean levels at study end. Significant increases or decreases are outlined. Error bars are given for a 95% confidence interval.
At baseline, all patients who were classified to be hypogonadal according the ADAM score answered at least one of the questions “1” and “7” with yes (Figure 2). At the end of the study, only 15% of patients were classified as hypogonadal in the ADAM questionnaire in the TRT group. In the CTR group, this percentage did not change. In the EU group, this percentage of patients rose significantly (p=0.01) from 0% at baseline to 37% (n=8) at study end.
3.5 Sexual function
TRT patients reported a median of 7 annual sexual activities at baseline, CTR patients reported a median of 6 and patients in the EU group reported significantly more annual sexual activities (15, p<0.001) at baseline. During the first year of treatment, the number of annual sexual activities among TRT patients rose significantly to 29 (p<0.001) and was significantly higher at the 12 months follow up visit among TRT patients not only compared to CTR patients (6 vs. 29, p<0.001) but also to the EU patients (16 vs. 29, p=0.05). After 5 years, the number of annual sexual activities remained stable and was significantly higher with 25 as compared to CTR and EU patients (CTR: 5, p<0.001; EU: 14, p<0.001) (Figure 5).
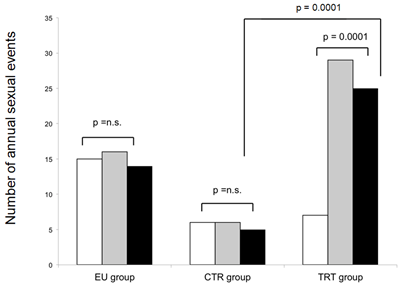
Figure 5: Annual sexual activities at baseline (white), after one year (dark grey) and five years (light grey bars) in absolute numbers.
3.6 Urological follow up
PSA was measured every 6 months in patients receiving TRT. 13% of the included patients developed a PSA elevation of 10.1 + 3.9 %. The consecutively performed IPSS score showed no signs for hyperplasia of the prostate. Routinely performed urological exams revealed no signs for prostate hyperplasia or cancer. None of the patients in the CTR and in the EU group developed prostate hyperplasia or signs for urogenital cancer.
3.7 Safety
There were no treatment-related patient drop-outs or differences with respect to serious adverse events between the three groups. Annual rejection episodes (1.2 ± 1, CTR group; 1.3 ± 1, TRT group; 1.1 ± 1, EU group) and hospitalization rates (2.4 ± 1.7, CTR group; 2.3 ± 1.8, TRT group; 2.2 ± 1.6 EU group) were similar in all groups. Study medication dose was not altered in any patient who had a rejection episode. Kidney function and blood count remained stable.
4. Discussion
To date, hypogonadism and bone health in patients after heart transplantation are not recognized as major problems for long term morbidity. Therefore, they are no dedicated treatment goals in long term care following CTX. Our current results however very clearly show a high burden of disease with a high verbetral fracture rates despite bisphosphonate, calcium and vitamin D therapy. Besides, we found a high risk for hypogonadism in 52 male patients several years after heart transplantation: 60% of the study participants were hypogonadal and the incidence for new vertebral fractures over the 5-year study period was high. At study start, bone health was significantly worse in hypogonadal compared to eugonadal men with lower BMD and more prevalent vertebral fractures. Although lower testosterone levels in CTX recipients are associated with greater bone loss, prospective studies on the effects of testosterone supplementation are scarce. With the present study we are able to provide important data regarding the effects of testosterone supplementation on top of bisphosphonate treatment in patients following heart transplantation screened for prostate pathology before study entry.
In a study by Fleischer et al., serum testosterone and gonadotropins were measured in cardiac transplant recipients receiving either calcitriol or alendronate in the first year after transplantation and no relationship between gonadotropin status and bone loss was observed. However, this study lacked a control group consisting of eugonadal patients and was performed in the early posttransplantation period [17]. Long term survivors after CTX seem to have a higher risk of bone loss and hypogonadism. Testosterone deficiency worsens bone resorption and leads to low BMD and increased fracture risk [29].
Various studies have taken different approaches to evaluate the importance of gonadal status for prevention of bone loss and fractures in CTX patients. In a small cross-sectional analysis, a negative correlation between sex hormone binding globuline and the femoral neck BMD was found without difference in fracture incidence [30]. It has been discussed that relatively high or even supraphysiological serum testosterone levels may be necessary to increase BMD [31]. The current study demonstrates that testosterone in a physiological dose does indeed have a beneficial effect on bone mass and is able to significantly reduce fracture rates. Besides, testosterone replacement also exerted beneficial effects on sexual function. Among patients who were classified as eugonadal at study entry, the percentage of patients who scored a positive ADAM test increased significantly from 0% at baseline to 37% (n=8) at study end. On the other hand, hypogonadal men who reported libido loss at study entry developed a normal libido with TRT throughout the study. Compared to a report on sexual function in patients with metabolic disorders and hypogonadism, libido of treated hypogonadal patients at study end was comparable to those reported for healthy men [32]. Interestingly, free plasma testosterone levels seem to be an independent predictor for vasculopathy of the allograft. Recently, low testosterone levels were identified as a novel risk factor for rejection after cardiac transplantation in men [33]. In our cohort however, we did not find a significant difference between groups for the frequency of rejection episodes.
Safety data on long term testosterone treatment are scarce-even in general healthy populations [34]. In patients who are receiving immunosuppressive therapy, the risk for malignancy may be greater and many clinicians may be reluctant to use testosterone in this special cohort of patients. In our study, we found no urological adverse events - including however only patients after an urologic exam - 13% had to be excluded because of prostatic hyperplasia or suspected cancer identified at urological assessment.
Our study has several limitations. One is the fact that we included only male patients at least one year after heart transplantation. The effect of testosterone replacement earlier after transplantation and the effect of sex hormone replacement in women may be different. However, premenopausal women who would be candidates for estrogen replacement are the exception in cardiac transplantation populations and often receive hormonal contraception. All patients at our institution receive life long triple immunosuppressive therapy including steroids-in the present study with an average daily prednisolone dose above 6mg. This may be a reason not only for the high prevalence of hypogonadism but also the high fracture incidence despite bisphosphonate therapy [35]. Another limitation is the fact that hypogonadism was defined according to free testosterone levels-SHBG levels were not assessed.
In summary, the current data suggest that hypogonadism is an important contributor to bone loss and fracture risk among long term survivors of heart transplantation. To the best of our knowledge, this 5-year study is the first to show a beneficial effect of testosterone supplementation on top of standard osteoporosis therapy not only on BMD but also on fracture risk and sexual function in hypogonadal heart transplanted men. Our data from a relatively small sample call for larger clinical trials in transplanted hypogonadal patients with osteoporosis to confirm the benefits of testosterone replacement therapy.
Acknowledgements
The authors thank Matthias Wagner for data bank management and Lisa Stach and her team for laboratory analyses. Study design: AFP, HD, HPD; Study conduct: DW, GP and AFP; Data collection: DW, GP, KA, SP, AJT, TRP, AFP; Data analysis: DW, SP AJT, AFP; Data interpretation: DW, HD, TRP, AFP; Drafting manuscript: DW, GP, KA and AFP. Revising manuscript content: HPD, AJT, SP; TRP, Approving final version of manuscript: DW, GP, HD, HPD, KA, AJT, SP, TRP, AFP; AFP takes responsibility for the integrity of the data analysis.
References
- Shah KB, Parameshwat J. Advances in heart transplantation: The year in review. J Heart Lung Transplant 30 (2011): 241-245.
- Van Cleemput J, Daenen W, Nijs J Timing and quantification of bone loss in cardiac transplant recipients. Transplant Int 8 (1995): 196.
- Sambrook PN, Kelly PJ, Fontana D. Mechanism of rapid bone loss following cardiac transplantation Osteoporos Int 4 (1994): 273.
- Fahrleitner-Pammer A, Piswagner-Sölkner JC, Pieber TR, et al. Ibandronate prevents bone loss and reduces vertebral fracture risk in male cardiac transplant patients: a randomized double blind placebo controlled trial. J Bone Miner Res 24 (2009): 1335-1344.
- Ledig-Bruckner G, Hosch S, Dodidou P, et al. Frequency of the osteoporotic fractures after cardiac transplantation: A follow up study. Lancet 357 (2001): 342-347.
- Ebeling PR. Approach to the patient with transplantation-related bone loss- J Clin Endocrinol Metab. 94 (2009): 1483-1490.
- Fahrleitner A, Prenner G, Leb G, et al. Serum osteoprotegerin is a major determinant of bone density development and prevalent vertebral fracture status following cardiac transplantation. Bone 32 (2003): 96-106.
- Berguer DG, Krieg MA, Thiébaud D. Osteoporosis in heart transplant recipients: A longitudinal study. Transplant Proc 26 (1994): 26-49.
- Carbonare LD, Zanatta M, Braga V, et al. Densitometric treshold and vertebral fractures in heart transplant patients. Transplantation 92 (2011): 106-111.
- Lindsay R. Bone loss after cardiac transplantation. N Engl J Med 350 (2004): 751-754.
- Cattabiani C, Basaria S, Ceda GP, et al. Relationship between testosterone deficiency and cardiovascular risk and mortality in adult men. J Endocrinol Invest. 35 (2012): 104-120.
- Bours SP, van Geel TA, Geusens PP, et al. Contributors to secondary osteoporosis and metabolic bone disease in patients presenting with a clinical fracture. J Clin Endocrinol Metab 96 (2011): 1360-1367.
- Isaia G, Mussetta M, Pecchiio F, et al. Effect of testosterone on bone in hypogonadal males 15 (1992): 47-51.
- Morley JE, Perry HM 3d, Kaiser FE, et al. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc 41 (1993): 149-152.
- Mellström D, Johnell O, Ljunggren O. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden J Bone Miner Res 21 (2006): 529-535.
- Meier C, Liu PY, HAndelsman DJ, Seibel MJ Endocrine regulation of bone turnover in men. Clin Endocrinol (Oxf.) 63 (2005): 4789-4796.
- Fleischer J, McMahon DJ, Hembree W, et al. Serum testosterone levels after cardiac transplantation Transplantation 85 (2008): 834-839.
- Shane E, Rivas M, McMahon DJ. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 82 (1997): 1497.
- Kenny AM, Prestwood KM, Raisz LG. Short term effects of intramuscular and transdermal testosterone on bone turnover, prostate symptoms, cholesterol and hematocrit in men over the age of 70 with low testosterone levels. Endocrine Research 26 (2000): 153-168.
- Meier C, Nguyen TV, Handelsman DJ, et al. Endogenous sex hormones and incident fracture risk in older men The Dubbo Osteoporosis Epidemiology Study. Arch Intern Med 168 (2008): 47-57.
- Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8 (1993): 1137.
- Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty fourth official adult heart report 2007. J Heart Lung Transplant 26 (2007): 769-781.
- Schweiger M, Wasler A, Prenner G, et al. The prognostic validity of ventricular evoked response (VER) signals in cardiac transplantation J Heart Lung Transplant 24 (2005): 1730-1735.
- Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8 (1993): 1137-1148.
- Gray A, Feldman HA, Mc Kinlay JB. Age, disease and changing sex hormone levels in middle aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 73 (1991): 1016-1025.
- Wagner D, Amrein K, Dimai HP, et al. Ibandronate and calcitrol reduces fracture risk, reverses bone loss and normalizes bone turnover after LTX. Transplantation 93 (2012): 331-336.
- Valimaki MJ, Kinnunen K, Tahtela R, et al. A prospective study of bone loss and turnover after cardiac transplantation: Effect of calcium supplementation with or without calcitonin. Osteoporos Int 10 (1999): 128-136.
- Erben RG, Brunner KS, Breig B, et al. Skeletal effects of cyclosporine A are gender related in rats. Endocrinology 144 (2003): 40-49.
- Behre HM, Kleisch S, Leifke E, et al. Long term effect of testosterone therapy on bone mineral denisty in hypogonadal men. J Clin Endocrinol Metab 82 (1997): 2386-2390.
- Hoefle G, Tautermann G, Saely CH, et al. Sex hormone binding globulin is negatively correlated with femoral bone mineral density in male cardiac transplant recipients. Wien Klin Wochenschr 116 (2004): 170-175.
- Behre HM, Oberpenning F, Nieschlag E. Comparative pharmacokinetics of testosterone
- preparations: application of computer analysis and simulation. In Nieschlag E, Behre HM, eds. Testosterone-action, deficiency, substitution. Berlin, Heidelberg, New York: Springer (1990): 115-135.
- Shelton JB, Rajfer J. Androgen deficiency in aging and metabolically challenged men. Urol Clin North Am 39 (2012): 63-75.
- Caretta N, Feltrin G, Tarantini G, et al. Low serum testosterone as a new risk factor for chronic rejection in heart transplanted men. Transplantation 1596 (2013): 501-505.
- William J, Bremner M. Testosterone deficiency and replacement in older men. N Engl J Med 363 (2010): 189-191.
