Does Gender Contribute to Any Differences in Caries and Periodontal Status in UK Children?
Article Information
Sofia Papadaki1, Ioannis Pilalas2, Jing Kang3*
1Dental Core Trainee at the James Cook University Hospital, Middlesbrough, UK 2Defence Primary Healthcare, Dental Centre Catterick ITC, Catterick Garrison, UK 3School of Dentistry, University of Leeds, Leeds, UK
*Corresponding Author: Jing Kang, School of Dentistry, University of Leeds, LS2 9LU, Leeds, UK
Received: 15 July 2022; Accepted: 29 July 2022; Published: 28 September 2022
Citation: Sofia Papadaki, Ioannis Pilalas, Jing Kang. Does Gender Contribute to Any Differences in Caries and Periodontal Status in UK Children?. Archives of Internal Medicine Research 5 (2022): 463-474.
View / Download Pdf Share at FacebookAbstract
Background: Gender Inequalities In Dental Caries And Periodontal Diseases Have Been Reported Among Adults. However, Literature Focusing On Children Is Scarce. The Aim Was To Examine Whether Gender Affects Caries Experience And Periodontal Status In UK Children Based On The 2013 Children’s Dental Health Survey Dataset (CDHS).
Methods: CDHS Included Children Aged 5, 8, 12 And 15 Years. Dental Caries Experience And Periodontal Status Were Reported Using The Number Of Decayed, Missing And Filled Teeth (DMFT Or Dmft For Permanent Or Primary Dentition At D1 And D3 Thresholds) And The Basic Periodontal Examination (BPE) Score. To Assess Gender Inequalities On DMFT/Dmft And BPE, Zero- Inflated Negative Binomial (ZINB) And Multinomial Logistic Regression Models Were Employed Respectively.
Results: Analyses Included 9,866 Children. No Gender Inequalities In Caries Experience Were Observed For The 5 And 8-Year-Old Groups. However, In 12- And 15-Year-Old Adolescents, Females Had Higher D3MFT Scores Compared To Males (IRR: 1.28, 95% CI: 1.10-1.49 And IRR: 1.16, 95% CI: 1.00-1.35). 15-Year-Old Females Had Lower Probability To Be Caries Free (OR: 0.59, 95% CI: 0.45-0.82). With Regards To Periodontal Status, Significant Gender Inequalities Were Not Observed (P>0.05).
Conclusions: In The UK, Female Adolescents Experienced More Carious Lesions Compared To Males When Dental Caries Was Diagnosed Into Dentine (D3MFT). However, 15-Year-Old Males Matched Females In Caries Experience When Early Enamel Lesions Were Considered (D1MFT). No Gender Dissimilarity Was Identified Among British Adolescents Regarding Periodontal Status. The Increased Dental Caries Risk Of Adolescent Females May Indicate The Necessity For Bespoke Oral Health Measures.
Keywords
Caries; Periodontal; Gender Inequality; Children’s Dental Health Survey
Caries articles; Periodontal articles; Gender Inequality articles; Children?s Dental Health Survey articles
Article Details
1. Introduction
Gender inequalities in dental caries and periodontal diseases have been widely investigated for adult populations. Many studies showed that females experience a higher burden of dental caries [1], while males were found to be more susceptible to exhibiting advanced periodontal disease [2]. However, evidence in the field of paediatric dentistry remains unclear since various studies had mixed conclusions. Some studies depicted gender bias regarding caries experience for juvenile patients in which females were reported to be more susceptible [3-8], while other studies supported an opposite trend, in which males especially at the primary dentition stage, exhibited higher caries experience than females of same age [9-12]. On the other hand, certain studies reported insignificant (p>0.05) gender inequalities in caries experience between genders [13-16].
For periodontal diseases, some literature suggested that male teenagers were more likely to demonstrate suboptimal oral hygiene [17-20] and develop gingivitis. However, some studies reported absence of significant gender inequalities in the periodontal status of children [21, 22]. It is important to underline that evidence on gender inequalities is limited due to biased sampling, small sample size and methodological flaws. This study aims to investigate whether gender impacts on differences in dental caries and periodontal status of children by using the UK 2013 Children’s Dental Health Survey (CDHS) data.
2. Material and Methods
2.1 Data source
The 2013 CDHS dataset included children from England, Wales and Northern Ireland. Data were collected through clinical examinations by calibrated dental teams, questionnaires completed by 12- and 15-year-old pupils and parent questionnaires of participating children [23]. The target populations were the 5-, 8-, 12- and 15-year-old children, representing primary (5-year-old), mixed (8-year-old) and permanent dentition (12- and 15-year-old) [23]. Ethical approval for CDHS was gained by the ethics committee of University College London (Project ID 2000/003) and participants had consented in advance for data use in future research projects.
2.2 Outcome measures
There were two outcome measures:
- Dental caries experience based on DMFT/dmft scores
- Periodontal status using BPE scores
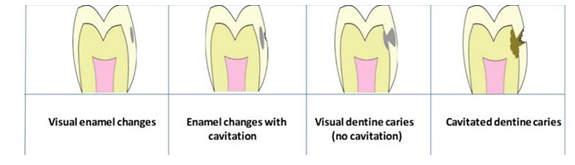
Dental caries experience was measured at two distinct threshold including enamel lesions (D1) and dentinal lesions (D3). Figure 1 demonstrates the differences between those thresholds. Periodontal status was measured based on BPE scores (Table 2), which were only available for 15-year-old children.
|
D1MFT/d1mft |
D3MFT/d3mft |
|
Decayed teeth including early enamel lesions along with dentinal caries |
Decayed teeth, including caries lesions into dentine only |
|
Missing teeth due to caries |
Missing teeth due to caries |
|
Filled teeth |
Filled teeth |
Table 1: Explanation of differences between D1MFT/d1mft (enamel lesions) and D3MFT/d3mft (dentine lesions).
|
Levels |
BPE scores |
|
|
Level 1 |
0 |
No bleeding |
|
Level 2 |
1 |
Bleeding on probing |
|
Level 3 |
2 |
Bleeding with retentive factors, such as calculus |
|
Level 4 |
3 |
Bleeding with shallow pockets between 3.5-5.5mm |
|
4 |
Deep pockets>5.5mm |
Table 2: BPE scores and levels in multiple regression analysis.
2.3 Exposure
Gender was the main exposure measure.
2.4 Confounding factors
Those included demographic characteristics such as:
- Age (scale)
- Socio-economic status (SES; quantiles 1-5) [24]
- Dietary habits such as consumption of water or food and drinks containing sugar including cakes, biscuits, sweets, fruit juice, smoothies, energy and fizzy drinks (less than once a day, more than twice a day)
- Oral hygiene practices such as tooth brushing frequency (less than once a day, more than twice a day), tooth flossing (yes, no)
- Pattern of dental attendance [25] (only when problems arise, routinely)
- Experience of orthodontic treatment (yes, no)
- Dental anxiety levels (low, medium, high) [26, 27]
Further factors were examined, including children’s experience of dental prevention (fissure sealants, fluoride varnish) and use of fluoride toothpaste. However, covariates that were available for a small number of participants or did not reach the defined significance level [28] were not included in multivariate analysis for the adjustment of ZINB and MLR models.
2.5 Statistical analysis
Descriptive statistics were performed for comparisons between males and females. The zero- inflated negative binomial models (ZINB) were used to fit D1MFT/d1mft and D3MFT/d3mft scores, as indicated by the DMFT/dmft distribution. The ZINB model is a two-part model, of which the zero-inflated part estimates the chance of excessive zeros, and the count model estimates the number of DMFT/dmft. Confidence interval (CI) was set to 95% after sampling weight. Adjustments for demographic features, oral hygiene practices, dietary habits and dental anxiety were included in the models gradually. This led to three statistical models from a univariate to a multivariate analysis. The m0 model was unadjusted and compared the D1MFT/d1mft and D3MFT/d3mft differences between genders without taking into consideration other possible confounders.
The m1model was adjusted for demographic features. The m2, which applied only for the D1MFT and D3MFT variables, was adjusted for the above- mentioned covariates, including children’s SES, dental behaviour, dietary habits and dental anxiety. Subgroup statistical analysis to estimate children’s caries experience in each age group was performed. The subgroup analysis referred to 5-, 8-, 12- and 15- year-old children, respectively. With regards to periodontal status, data were analysed using the Multinomial Logistic Regression (MLR) model at four different levels [level 1, or reference level (BPE:0), level 2 (BPE:1), level 3 (BPE:2) and level 4 (BPE:3 or 4)]. The aim was to compare each group (levels 2, 3, 4) with the reference level (Table 2). The model was sequentially adjusted for SES, oral hygiene practices, including frequency of toothbrushing, flossing, dental attendance pattern, and experience of orthodontic treatment [29]. Data were weighed according to primary sampling unit (PSU) and strata, and then, analysis was performed using R studio with Survey Package and StataSE 14.
3. Results
In total, 9,866 children were included in analyses. A comparison of different covariates between genders is presented in Table 3. For dental caries, no differences were found among 5- and 8-year-old children at either caries threshold. Females were associated with higher DMFT scores, regardless of the threshold at which dental caries was diagnosed in 12-year-old children [IRR (D3): 1.28, 95%CI (1.10- 1.49), p<0.01 and IRR(D1): 1.22, 95% CI (1.10-1.35), p<0.001]. Discrepancies in caries experience between genders were evident among 15-year-old children, only at the dentinal threshold [IRR (D1): 1.16, 95% CI (1.00-1.35), p<0.05]. Also, the
possibility for 15-year-old females to be “caries-free” (DMFT:0) was lower compared to males [OR (D3):0.59, 95% CI (0.45-0.82), p<0.01 and OR
(D1):0.55, 95% (0.41-0.74), p<0.001], regardless of threshold. However, when caries experience included early enamel lesions (D1MFT), males matched females in caries experience (Table 4). The MLR model results, which compared periodontal status of 15-year-old females and males, are summarised in Table 5. There was no significant difference (p>0.05) for the examined BPE scores between genders.
|
Male |
Female |
p-value |
|
|
N |
4812 (48.8) |
5054 (51.2) |
|
|
Demographics |
|||
|
Age group |
0.496 |
||
|
5-year-old |
1264 (26.3) |
1285 (25.4) |
|
|
8-year-old |
1171 (24.3) |
1196 (23.7) |
|
|
12-year-old |
1222 (25.4) |
1310 (25.9) |
|
|
15-year-old |
1155 (24.0) |
1263 (25.0) |
|
|
HMRC quintile |
0.001 |
||
|
1 (Most deprived) |
1432 (30.9) |
1669 (34.5) |
|
|
2 |
1062 (22.9) |
1144 (23.7) |
|
|
3 |
952 (20.6) |
903 (18.7) |
|
|
4 |
710 (15.3) |
671 (13.9) |
|
|
5 (Least deprived) |
473 (10.2) |
448 (9.3) |
|
|
Oral hygiene practices |
|||
|
Frequency of brushing teeth |
0.001 |
||
|
Twice a day or more |
1652 (80.8) |
1765 (85.1) |
|
|
Less than twice a day |
393 (19.2) |
310 (14.9) |
|
|
Used dental floss in the last year = No |
1000 (80.6) |
1013 (76.1) |
0.006 |
|
Pattern of dental attendance = Only when in trouble/never |
428 (18.3) |
432 (17.1) |
0.28 |
|
Orthodontic treatment experience |
|||
|
Ever had bracket fitted or adjusted = No |
1493 (88.9) |
1446 (83.1) |
<0.001 |
|
Dietary habits |
|||
|
Frequency of eating cakes or biscuits |
0.018 |
||
|
Twice or more a day |
1040 (44.8) |
1024 (40.5) |
|
|
Once or less a day |
1280 (55.2) |
1506 (59.5) |
|
|
Frequency of eating sweets |
0.586 |
||
|
Twice or more a day |
966 (41.6) |
1074 (42.4) |
|
|
Once or less a day |
1355 (58.4) |
1460 (57.6) |
|
|
Frequency of drinking soft drinks containing sugar |
<0.001 |
||
|
Twice or more a day |
854 (37.1) |
845 (33.5) |
|
|
Once or less a day |
1451 (62.9) |
1674 (66.5) |
|
|
Frequency of drinking energy drinks |
<0.001 |
||
|
Twice or more a day |
416 (18.1) |
354 (14.2) |
|
|
Once or less a day |
1886 (81.9) |
2141 (85.8) |
|
|
Frequency of drinking fizzy drinks |
<0.001 |
||
|
Twice or more a day |
1103 (47.3) |
1062 (41.8) |
|
|
Once or less a day |
1225 (52.7) |
1476 (58.2) |
|
|
Frequency of drinking water |
0.049 |
||
|
Twice or more a day |
1631 (70.7) |
1810 (71.9) |
|
|
Once or less a day |
675 (29.3) |
706 (28.1) |
|
|
Dental anxiety |
|||
|
Self-rated dental anxiety score |
<0.001 |
||
|
Low/no anxiety |
935 (40.9) |
576 (23.4) |
|
|
Moderate anxiety |
1172 (51.2) |
1461 (59.5) |
|
|
Extreme anxiety |
181 (7.9) |
420 (17.1) |
|
|
Dental health outcomes |
|||
|
d3mft (mean (SD)) |
0.8 (1.8) |
0.7 (1.6) |
<0.001 |
|
D3MFT (mean (SD)) |
0.9 (1.9) |
1.1 (2.2) |
<0.001 |
|
d1mft (mean (SD)) |
1.2 (2.2) |
1.0 (2.1) |
0.001 |
|
D1MFT (mean (SD)) |
1.7 (3.0) |
1.9 (3.1) |
0.012 |
|
BPE (N %) |
0.849 |
||
|
0 (N %) |
530 (49.8) |
581 (51.4) |
>0.05 |
|
≠ 0 (N %) |
534 (50.2) |
550 (48.6) |
>0.05 |
Note: Data are presented as frequency (%) unless specified. Numbers may not sum to totals due to missing values; percentage
may not sum to 100 due to rounding.
Deprivation index was grouped in quintiles as follows:
1 (most deprived, ranks 1-6496), 2 (ranks 6497-12993), 3 (ranks 12994-19489), 4 (ranks 19490-25986), 5 (least deprived, ranks
25987-32482)
P-value was for comparing characteristics between male and female. SD: standard deviation
Table 3: Comparison between male and female participants in 2013 CDHS (n=9866).
|
Age (years) |
m0 |
m1 |
m2 |
||||||
|
5 |
NB, IRR (95% CI) |
ZI, OR (95% CI) |
NB, IRR (95% CI) |
ZI, OR (95% CI) |
NB |
ZI |
|||
|
d3mft |
0.89 (0.74, |
1.08 |
(0.90, |
0.90 (0.82, 1.10) |
1.07 (0.82, |
||||
|
1.00) |
1.35) |
1.35) |
|||||||
|
d1mft |
0.94 (0.82, |
1.09 |
(0.90, |
0.95 (0.82, 1.10) |
1.07 (0.90, |
||||
|
1.10) |
1.35) |
1.35) |
|||||||
|
8 |
NB, IRR (95% CI) |
ZI, OR (95% CI) |
NB, IRR (95% CI) |
ZI, OR (95% CI) |
NB |
ZI |
|||
|
d3mft |
0.93 (0.82, |
1.12 |
(0.90, |
0.91 (0.82, |
1.08 (0.90, |
||||
|
1.00) |
1.35) |
1.00). |
1.35) |
||||||
|
d1mft |
0.96 (0.90, |
1.17 |
(0.90, |
0.95 (0.90, 1.00) |
1.16 (0.90, |
||||
|
1.00) |
1.35) |
1.49) |
|||||||
|
12 |
NB, IRR (95% CI) |
ZI, OR (95% CI) |
NB, IRR (95% CI) |
ZI, OR (95% CI) |
NB, IRR (95% CI) |
ZI, OR (95% CI) |
|||
|
D3MFT |
1.28 (1.10, |
0.95 |
(0.74, |
1.25 (1.10, 1.49) |
1.11 (0.82, |
1.28 (1.10, |
0.96 (0.67, |
||
|
1.49) *** |
1.22) |
** |
1.49) |
1.49) ** |
1.35) |
||||
|
D1MFT |
1.12 (1.00, |
1.07 |
(0.82, |
1.12 (1.10, 1.22) |
1.28 |
1.22 (1.10, |
1.32 (0.90, |
||
|
1.22) * |
1.49) |
* |
(0.90, 1.82) |
1.35) *** |
1.35) |
||||
|
15 |
NB, IRR (95% CI) |
ZI, OR (95% CI) |
NB, IRR (95% CI) |
ZI, OR (95% CI) |
NB, IRR (95% CI) |
ZI, OR (95% CI) |
|||
|
D3MFT |
1.13 (1.00, |
0.70 |
(0.55, |
1.13 (1.00, 1.22) |
0.76 |
(0.55, |
1.16 (1.00, |
0.59 (0.45, |
|
|
1.22) * |
0.90) * |
* |
1.00) . |
1.35) * |
0.82) ** |
||||
|
D1MFT |
0.97 (0.90, |
0.64 |
(0.50, |
0.95 (0.90, 1.00) |
0.67 |
(0.50, |
1.01 (0.90, |
0.55 (0.41, |
|
|
1.10) |
0.90) ** |
0.90) * |
1.10) |
0.74) *** |
|||||
NB: Negative binomial part; Mean DMFT/dmft (2003/2013) difference between females and males with caries experience (females vs males); ZI: Zero-inflated part; Possibility of being caries free (females vs males); IRR: Incidence rate ratio; OR: Odds ratio; CI: Confidence interval; m0: Unadjusted model; m1: Adjusted model for demographic factors (SES); m2: Adjusted model for demographic features (SES), dental behaviour (pattern of dental attendance and toothbrushing), dietary habits (frequency of eating cakes or biscuits, drinking fizzy drinks, energy drinks, soft drinks containing sugar and water) and dental anxiety
. p value < 0.1; *p value < 0.05; **p value < 0.01; ***p value < 0.001
Table 4: Dental caries experience between females and males (females vs males).
|
BPE |
m0RRR (95% CI) |
m1RRR (95% CI) |
m2RRR (95% CI) |
|
0 (ref) |
1 |
1 |
1 |
|
1 |
0.90 (0.74, 1.10) |
0.87 (0.70, 2.07) |
1.22 (0.74, 2.01) |
|
2 |
0.98 (0.82, 1.22) |
0.79 (0.74, 1.10) |
0.62 (0.33, 1.10) |
|
3 or 4 |
1.03 (0.67, 1.65) |
0.90 (0.61, 1.35) |
0.35 (0.12, 1.97) . |
RRR: Relative risk ratio; CI: Confidence interval; m0: Unadjusted model; m1: Adjusted model for demographic factors (SES);
m2: Adjusted model for demographic features and dental behaviour including pattern of dental attendance, frequency of tooth brushing, flossing and participants’ orthodontic treatment experience.
. p value < 0.1; *p value < 0.05; **p value < 0.01; ***p value < 0.001
Table 5: Periodontal status between the 15-year-old females and males (females Vs males).
4. Discussion
This is the first study that reported on gender inequalities using a large UK dataset which included enamel and dentine caries. Gender inequalities were not noted for primary dentition for dental caries nor for periodontal diseases. A potential explanation could be that children of such young age are not able to determine their dietary habits nor are responsible for daily oral hygiene. Therefore, the most important risk factors are influenced directly by parents or guardians, which could demarcate the absence of gender susceptibility to dental caries. Notably, most studies agree with the lack of significant gender discrepancy regarding dental caries experience in primary dentition [13, 16, 30, 31]. However, it would be prudent not to draw firm conclusions due to lack of data on eating habits and oral hygiene practices for those age groups. In the permanent dentition, the outcomes were more distinct as females in the 12- year-old group, had higher D1MFT and D3MFT scores. This susceptibility was also confirmed among 15-year-old adolescents, but only when dental caries was diagnosed into dentine (D3MFT). When early enamel lesions were included in caries diagnosis, the 15-year-old males matched females in caries experience, which highlights that caries experience for males comprised of more enamel lesions. Therefore, gender discrepancy at younger ages or when caries examined into dentine only, may be attributed to earlier tooth eruption in girls.
According to pupil questionnaires, female adolescents consumed sweets and sugary soft drinks less frequently, while they brushed their teeth more frequently. Ideally, this should have materialised in less exposure to dental caries. However, higher caries prevalence among females may be associated with erroneous assumptions on healthy and balanced diet from a dental perspective. It could be hypothesised that participants may have underestimated the daily consumption of hidden sugars with exponential cariogenic risk [32-34]. Another explanation of higher female susceptibility may be associated with earlier tooth eruption and, therefore, longer exposure to cariogenic products in the oral cavity [35]. Furthermore, some genome-wide-association-studies (GWAS) asserted that gender differences may be attributed to several gene variations influencing the oral environment, enamel formation, dietary preferences and composition of pathogenic bacteria [1, 36, 37]. Additionally, differences in saliva composition and in overall saliva flow rate could stem from hormonal changes due to puberty onset [38], pregnancy or menstruation, which in turn, renders female adolescents more prone to dental caries [10, 39]. Among physiological variations between genders, elevated oestrogen levels in females have been associated with changes in saliva composition and reduction in salivary flow. These conditions in combination with a cariogenic diet could contribute to caries incidence among female adolescents [40].
Although there is no consensus regarding the existence of specific gender inequalities in caries experience for the permanent dentition of adolescents, a few studies [3, 5, 8, 41] were in accordance with our findings. Multiple studies [21, 22, 42] agreed with the absence of gender discrepancies regarding periodontal status of the 15- year-old adolescents based on BPE scores which was also affirmed by our findings. In contrast, other studies [9, 10, 13, 14] reported that males had worse gingival or periodontal status than females. The reported susceptibility of male adolescents to poorer periodontal/gingival status may stem from suboptimal oral hygiene regime rather than genetic superiority of females. Also, notable deviations in study design, sample selection and indices do not allow direct comparisons to be made or concrete conclusions to be drawn.The high-quality 2013 CDHS dataset offers the opportunity for comprehensive analysis of children’s oral health [15]. Furthermore, the different thresholds at which dental caries was diagnosed are reflected eloquently in the present study, eliminating the risk of confusion or result deviations and allowing comparisons between different outcomes. Another strength of this study is reflected on the ΖΙΒΝ model used to investigate caries experience, which is the most reliable and appropriate statistical approach according to DMFT/dmft distribution [43].
However, there are also certain limitations. A number of factors may mean that CDHS underestimates the true population levels of oral disease. As with most surveys, CDHS was based solely on clinical examination, and epidemiological convention calibrates examiners to “underdiagnose” the presence of dental caries in case of uncertain diagnosis [15]. Also, BPE may underestimate periodontal disease [44]. Additionally, children attending special needs schools, where oral disease levels may be higher, were not included in CDHS which may have underestimated the burden of such oral diseases among British children [45, 46]. A further limitation is that self-reported dietary habits for the 12- and 15- year-old groups may have underestimated the consumption of cariogenic food and drinks. Limited availability of dietary data may mean that the ZINB model was not adjusted for those factors for 5- or 8- year-old groups. As a result, estimations of d3mft and d1mft may have been more susceptible to errors. Furthermore, BPE data were only available for the 15-year-old group, rendering comparisons between age groups impossible.
Despite our findings, more prospective studies are required to ascertain accurate conclusions before additional preventative measures can be recommended based on gender and further investigation on the impact of individual regional water fluoridation scheme may play in children’s caries experience would be useful. This investigation could reflect the need for further preventative measures, such as the implementation of water fluoridation schemes in more communities across the UK. With respect to children’s periodontal status, more prospective studies that adopt a widely agreed index, would be useful in investigating the potential for advanced periodontal treatment needs among children of different ages.
References
- Lukacs Sex differences in dental caries experience: clinical evidence, complex etiology. Clin Oral Investig 15 (2011): 649- 656.
- Shiau HJ, Reynolds Sex differences in destructive periodontal disease: a systematic review. J Periodontol 81 (2010): 1379-1389.
- Brito ACM, Bezerra IM, Cavalcante DdFB, et Dental caries experience and associated factors in 12-year-old-children: a population based-study. Brazilian Oral Research 34 (2020).
- Huew R, Waterhouse PJ, Moynihan PJ, et Prevalence and severity of dental caries in Libyan schoolchildren. Int Dent J 61 (2011): 217-223.
- Oliveira LBd, Moreira RdS, Reis SCGB, et Dental caries in 12-year-old schoolchildren: multilevel analysis of individual and school environment factors in Goiânia. Revista Brasileira de Epidemiologia 18 (2015): 642-654.
- Perera PJ, Abeyweera NT, Fernando MP, et Prevalence of dental caries among a cohort of preschool children living in Gampaha district, Sri Lanka: a descriptive cross sectional study. BMC Oral Health 12 (2012): 49.
- Shaffer JR, Wang X, McNeil DW, et Genetic Susceptibility to Dental Caries Differs between the Sexes: A Family-Based Study. Caries Research 49 (2015): 133-140.
- Veerasamy A, Kirk R, Gage Epidemiology of dental caries among adolescents in Tamil Nadu, India. Int Dent J 66 (2016): 169-177.
- Vanobbergen J, Martens L, Lesaffre E, et Assessing risk indicators for dental caries in the primary dentition. Community Dent Oral Epidemiol 29 (2001): 424-434.
- Shaffer JR, Leslie EJ, Feingold E, et Caries Experience Differs between Females and Males across Age Groups in Northern Appalachia. International Journal of Dentistry 2015 (2015): 938213.
- Mantonanaki M, Hatzichristos T, Koletsi- Kounari H, et Socio-demographic and area-related factors associated with the prevalence of caries among preschool children in Greece. Community Dent Health 34 (2017): 112-117.
- Abbass MMS, Mahmoud SA, El Moshy S, et The prevalence of dental caries among Egyptian children and adolescences and its association with age, socioeconomic status, dietary habits and other risk factors. A cross- sectional study. F1000Res 8 (2019): 8.
- Casanova-Rosado AJ, Medina-Solís CE, Casanova-Rosado JF, et Dental caries and associated factors in Mexican schoolchildren aged 6-13 years. Acta Odontol Scand 63 (2005): 245-251.
- Saravanan S, Madivanan I, Subashini B, et Prevalence pattern of dental caries in the primary dentition among school children. Indian Journal of Dental Research 16 (2005): 140-146.
- Gorbatova MA, Gorbatova LN, Pastbin MU, et al. Urban-rural differences in dental caries experience among 6-year-old children in the Russian north. Rural Remote Health 12 (2012): 1999.
- Wyne Caries prevalence, severity, and pattern in preschool children. J Contemp Dent Pract 9 (2008): 24-31.
- Levin L, Margvelashvili V, Bilder L, et Periodontal status among adolescents in Georgia. A pathfinder study. PeerJ 1 (2013): e137-e137.
- Zhang S, Xu B, Liu J, et Dental and periodontal status of 12-year-old Dai school children in Yunnan Province, China: a cross-sectional study. BMC Oral Health 15 (2015): 117.
- Amran AG, Alhajj MN, Al-Rafik NA. Evaluation of Gingival Health Status among 6- and 12-years-old Children in Dhamar City, Yemen: A Cross-sectional J Contemp Dent Pract 17 (2016): 440-444.
- Elias-Boneta AR, Ramirez K, Rivas- Tumanyan S, et Prevalence of gingivitis and calculus in 12-year-old Puerto Ricans: a cross-sectional study. BMC Oral Health 18 (2018): 13.
- Vadiakas G, Oulis CJ, Tsinidou K, et Oral hygiene and periodontal status of 12 and 15-year-old Greek adolescents. A national pathfinder survey. Eur Arch Paediatr Dent 13 (2012): 11-20.
- Kissa J, Chemlali S, El Houari B, et Aggressive and chronic periodontitis in a population of Moroccan school students. Journal of Clinical Periodontology 43 (2016): 934-939.
- Anderson T, Thomas C, Ryan R, et Children's dental health survey 2013 technical report England, Wales and Northern Ireland. London: Health and Social Care Information Centre (2015).
- Reisine ST, Psoter Socioeconomic status and selected behavioral determinants as risk factors for dental caries. J Dent Educ 65 (2001): 1009-1016.
- Angelillo IF, Anfosso R, Nobile CG, et Prevalence of dental caries in schoolchildren in Italy. Eur J Epidemiol 14 (1998): 351- 357.
- Akbay Oba A, Dülgergil CT, Sönmez Prevalence of dental anxiety in 7- to 11- year-old children and its relationship to dental caries. Med Princ Pract 18 (2009): 453-457.
- Milsom KM, Tickle M, Humphris GM, et The relationship between anxiety and dental treatment experience in 5-year-old children. Br Dent J 194 (2003): 503-506.
- Wasserstein RL, Schirm AL, Lazar Moving to a World Beyond “p < 0.05”. The American Statistician 73 (2019): 1-19.
- Ristic M, Vlahovic Svabic M, Sasic M, et Clinical and microbiological effects of fixed orthodontic appliances on periodontal tissues in adolescents. Orthod Craniofac Res 10 (2007): 187-195.
- Saravanan S, Kalyani V, Vijayarani MP, et Caries prevalence and treatment needs of rural school children in Chidambaram Taluk, Tamil Nadu, South India. Indian J Dent Res 19 (2008): 186-190.
- Kirthiga M, Murugan M, Saikia A, et Risk Factors for Early Childhood Caries: A Systematic Review and Meta-Analysis of Case Control and Cohort Studies. Pediatr Dent 41 (2019): 95-112.
- Guideline: sugars intake for adults and children. World Health Organization (2015).
- Marí Hidden sugar in food: A. risk for health. J Clin Nutr Diet 3 (2017): 1-3.
- Pitts NB, Zero DT, Marsh PD, et Dental caries. Nature reviews Disease primers 3 (2017): 1-16.
- Klein H, Palmer Studies on dental caries: VII. Sex differences in dental caries experience of elementary school children. Public Health Reports (1938): 1685-1690.
- Patir A, Seymen F, Yildirim M, et Enamel formation genes are associated with high caries experience in Turkish children. Caries Res 42 (2008): 394-400.
- Vieira AR, Marazita ML, Goldstein- McHenry Genome-wide scan finds suggestive caries loci. J Dent Res 87 (2008): 435-439.
- Rose SR, Municchi G, Barnes KM, et Spontaneous growth hormone secretion increases during puberty in normal girls and boys. J Clin Endocrinol Metab 73 (1991): 428-435.
- Martinez-Mier EA, Zandona The impact of gender on caries prevalence and risk assessment. Dent Clin North Am 57 (2013): 301-315.
- Lukacs JR, Largaespada Explaining sex differences in dental caries prevalence: saliva, hormones, and "life-history"etiologies. Am J Hum Biol 18 (2006): 540-555.
- Campus G, Solinas G, Cagetti MG, et National Pathfinder survey of 12-year-old Children's Oral Health in Italy. Caries Res 41 (2007): 512-517.
- Alonge OK, Narendran Periodontal health status of school children in St. Vincent and the Grenadines. Odontostomatol Trop 22 (1999): 18-22.
- Preisser JS, Das K, Long DL, et Marginalized zero-inflated negative binomial regression with application to dental caries. Stat Med 35 (2016): 1722- 1735.
- Bassani DG, da Silva CM, Oppermann Validity of the "Community Periodontal Index of Treatment Needs" (CPITN) for population periodontitis screening. Cad Saude Publica 22 (2006): 277-283.
- Oh TJ, Nam OH, Kim MS, et Oral Health of Patients with Special Health Care Needs After General Anesthesia: A 25- Year Retrospective Study. Pediatr Dent 40 (2018): 215-219.
- Zhou N, Wong HM, McGrath Oral health and associated factors among preschool children with special healthcare needs. Oral Dis 25 (2019): 1221-1228.
- Children’s Dental Health Survey Report 2: Dental Disease and Damage in Children. England, Wales and Northern Ireland (2015).