Dietary Patterns and Nutrient Intakes in SLE Patients in Oman
Article Information
Asma Al Kindi1, A'Shaima Al Kindi2, Batool Hassan3, Saied Al Yahyai4, Mostafa Waly5, Aliya Alansari1*
1Department of biology, College of Science, Sultan Qaboos University, Oman
2Department of Nutrition and Dietetics, Sultan Qaboos University Hospital, Oman
3Department of Medicine, College of Medicine and Health Sciences, Sultan Qaboos University, Oman
4Department of Genetics, College of Medicine and Health Sciences, Sultan Qaboos University, Oman
5Department of Food Science and Nutrition, College of Agriculture and Marine Sciences, Sultan Qaboos University, Oman
*Corresponding Author: Aliya Al Ansari, Department of Biology, College of Science, Sultan Qaboos University, Oman
Received: 07 April 2020; Accepted: 03 April 2020; Published: 22 April 2020
Citation: ffff
View / Download Pdf Share at FacebookAbstract
Systemic lupus erythematosus is an autoimmune disease with unexpected flares that is thought to be affected by dietary habits. In order to explore the dietary habits of Omani patients, we analyzed the intake of nutrients based on the assessment of diet from the semi-quantitative FFQ. Dietary patterns of sixty Omani cases and sixty age-gender-and origin- matched controls were assessed in terms of the recommended number of servings per day according to the Omani healthy plate for the main food groups and compared to the recommended dietary allowances (RDA) per day. Cases were found to consume proteins, fats and carbohydrates higher than RDA per day. Micronutrient intakes by cases showed that the intake of calcium, magnesium, iron, selenium, iodine, vitamins B6, D and E were lower than RDA, while phosphorous and vitamin B12 intake were higher. These observations were similar to previous studies except for B12 that was reported to be lower in SLE cases. However, this is considered as pilot study and the findings need to be confirmed by larger size study.
Keywords
Systemic Lupus Erythematosus, Dietary patterns, Nutrients intake, Semi-quantitative food frequency questionnaire, Oman
Systemic Lupus Erythematosus articles, Dietary patterns articles, Nutrients intake articles, Semi-quantitative food frequency questionnaire articles, Oman articles
Systemic Lupus Erythematosus articles Systemic Lupus Erythematosus Research articles Systemic Lupus Erythematosus review articles Systemic Lupus Erythematosus PubMed articles Systemic Lupus Erythematosus PubMed Central articles Systemic Lupus Erythematosus 2023 articles Systemic Lupus Erythematosus 2024 articles Systemic Lupus Erythematosus Scopus articles Systemic Lupus Erythematosus impact factor journals Systemic Lupus Erythematosus Scopus journals Systemic Lupus Erythematosus PubMed journals Systemic Lupus Erythematosus medical journals Systemic Lupus Erythematosus free journals Systemic Lupus Erythematosus best journals Systemic Lupus Erythematosus top journals Systemic Lupus Erythematosus free medical journals Systemic Lupus Erythematosus famous journals Systemic Lupus Erythematosus Google Scholar indexed journals Dietary patterns articles Dietary patterns Research articles Dietary patterns review articles Dietary patterns PubMed articles Dietary patterns PubMed Central articles Dietary patterns 2023 articles Dietary patterns 2024 articles Dietary patterns Scopus articles Dietary patterns impact factor journals Dietary patterns Scopus journals Dietary patterns PubMed journals Dietary patterns medical journals Dietary patterns free journals Dietary patterns best journals Dietary patterns top journals Dietary patterns free medical journals Dietary patterns famous journals Dietary patterns Google Scholar indexed journals Nutrients intake articles Nutrients intake Research articles Nutrients intake review articles Nutrients intake PubMed articles Nutrients intake PubMed Central articles Nutrients intake 2023 articles Nutrients intake 2024 articles Nutrients intake Scopus articles Nutrients intake impact factor journals Nutrients intake Scopus journals Nutrients intake PubMed journals Nutrients intake medical journals Nutrients intake free journals Nutrients intake best journals Nutrients intake top journals Nutrients intake free medical journals Nutrients intake famous journals Nutrients intake Google Scholar indexed journals Semi-quantitative food frequency questionnaire articles Semi-quantitative food frequency questionnaire Research articles Semi-quantitative food frequency questionnaire review articles Semi-quantitative food frequency questionnaire PubMed articles Semi-quantitative food frequency questionnaire PubMed Central articles Semi-quantitative food frequency questionnaire 2023 articles Semi-quantitative food frequency questionnaire 2024 articles Semi-quantitative food frequency questionnaire Scopus articles Semi-quantitative food frequency questionnaire impact factor journals Semi-quantitative food frequency questionnaire Scopus journals Semi-quantitative food frequency questionnaire PubMed journals Semi-quantitative food frequency questionnaire medical journals Semi-quantitative food frequency questionnaire free journals Semi-quantitative food frequency questionnaire best journals Semi-quantitative food frequency questionnaire top journals Semi-quantitative food frequency questionnaire free medical journals Semi-quantitative food frequency questionnaire famous journals Semi-quantitative food frequency questionnaire Google Scholar indexed journals Oman articles Oman Research articles Oman review articles Oman PubMed articles Oman PubMed Central articles Oman 2023 articles Oman 2024 articles Oman Scopus articles Oman impact factor journals Oman Scopus journals Oman PubMed journals Oman medical journals Oman free journals Oman best journals Oman top journals Oman free medical journals Oman famous journals Oman Google Scholar indexed journals vitamin B12 articles vitamin B12 Research articles vitamin B12 review articles vitamin B12 PubMed articles vitamin B12 PubMed Central articles vitamin B12 2023 articles vitamin B12 2024 articles vitamin B12 Scopus articles vitamin B12 impact factor journals vitamin B12 Scopus journals vitamin B12 PubMed journals vitamin B12 medical journals vitamin B12 free journals vitamin B12 best journals vitamin B12 top journals vitamin B12 free medical journals vitamin B12 famous journals vitamin B12 Google Scholar indexed journals healthy plate articles healthy plate Research articles healthy plate review articles healthy plate PubMed articles healthy plate PubMed Central articles healthy plate 2023 articles healthy plate 2024 articles healthy plate Scopus articles healthy plate impact factor journals healthy plate Scopus journals healthy plate PubMed journals healthy plate medical journals healthy plate free journals healthy plate best journals healthy plate top journals healthy plate free medical journals healthy plate famous journals healthy plate Google Scholar indexed journals dietary habits articles dietary habits Research articles dietary habits review articles dietary habits PubMed articles dietary habits PubMed Central articles dietary habits 2023 articles dietary habits 2024 articles dietary habits Scopus articles dietary habits impact factor journals dietary habits Scopus journals dietary habits PubMed journals dietary habits medical journals dietary habits free journals dietary habits best journals dietary habits top journals dietary habits free medical journals dietary habits famous journals dietary habits Google Scholar indexed journals autoimmune disease articles autoimmune disease Research articles autoimmune disease review articles autoimmune disease PubMed articles autoimmune disease PubMed Central articles autoimmune disease 2023 articles autoimmune disease 2024 articles autoimmune disease Scopus articles autoimmune disease impact factor journals autoimmune disease Scopus journals autoimmune disease PubMed journals autoimmune disease medical journals autoimmune disease free journals autoimmune disease best journals autoimmune disease top journals autoimmune disease free medical journals autoimmune disease famous journals autoimmune disease Google Scholar indexed journals
Article Details
Abbreviations
SLE-Systemic lupus erythematosus; FFQ-Food Frequency Questionnaire; SFFQ-Semi-Quantitative Food Frequency Questionnaire, RDA-Recommended daily allowances; AI-Adequate Intake
Introduction
Systemic lupus erythematosus (SLE) is a chronic, autoimmune disease characterized by autoantibodies production and immune complex deposition resulting in inflammation of some tissues and organs [1]. The disease runs with unpredictable course of flare and remission. There is an increasing interest in understanding the relationship between diet and SLE since there are significant differences in some vitamin levels between SLE patients and healthy people. Although diet alterations can reduce the risk of associated conditions such as atherosclerosis and metabolic syndrome [2], there is no clear evidences showing the effect of dietary changes in the SLE development and disease activity in humans [2].
1.1 Dietary patterns
Dietary pattern analysis for food items or food groups are important for examining the relationship between food or nutrients and the risk of chronic disease development [3]. Some studies linked the development of SLE to meat [4] and milk [5] consumption and classified them as strong and significant risk factor, whereas fish a weak and not significant risk factor [6]. Mediterranean diet, which is rich in vegetables, fruits, seeds, grains, nuts, two portions of fish per week and a small amount of meat and dairy, is recommended for SLE patients [7].
1.2 Nutrient Intakes
Nutrients are divided into macronutrients (carbohydrates, proteins and fat) and micronutrients (such as vitamins, selenium and iron). Specific nutrients were found to be associated with increasing SLE flares such as excess calories, fats, proteins, iron and zinc, while some micronutrients reported to have beneficial effects on SLE cases such as vitamin A, vitamin B, vitamin C, vitamin D, vitamin E, selenium, calcium [8]. For example, vitamin A level in SLE patients found to be significantly lower compared to healthy controls (p=0.001) [9]. Furthermore, they reported that 77.77% of the recruited patients had lower than 30 mcg/dl of vitamin A, i.e. hypovitaminosis. In animal models, vitamin A supplementation has shown to decrease SLE pathogenesis through the reduction of proteinuria and glomerulonephritis [10]. One of the potential mechanisms through which vitamin A can reduce pathogenesis is by modulating the Th17/ Treg balance by increasing Treg cells and decreasing Th17 cells and therefore reducing the inflammatory response. Handono et al. [11] found that the supplementation of CD4+ T cell cultures with vitamin A can modulate the ratio of Th17/ Treg [11].
In SLE patients, vitamin B complex intake reduces triglycerides and LDL-C and improves clinical symptoms [12] such as fatigue, fever and cardiovascular diseases [13]. In a study on Japanese SLE patients, increased doses of Vitamin B6 and folate were able to reduce disease severity regardless of the non-dietary factors [14]. Vitamin D deficiency is associated with severity and disease activity of SLE [15]. In addition, vitamin D has an immunomodulatory role on adaptive immune response by inhibiting Th1 and Th17 cells in humans and mice. It can also stimulate the differentiation of T cells towards Treg cells and maintain their induction [16].
1.3 Diet and microbiome
Dietary patterns are also important due to their influence on the microbiota diversity. For example, low fat/ high fiber diet was reported to be positively associated with Firmicutes levels [17, 18]. The gut microbiota helps the host in extracting and digesting nutrients from their diets, protect against enteropathogens and for normal immune functions and responses, especially the development of T helper (Th) cell lineages [19, 20]. Any significant change in the composition of microbiota may lead to autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease (IBD) and SLE [21, 22]. The influence of gut microbiota was reported by López et al. [23] when the differentiation of Th17 was increased in naïve T cells when co-cultured with stool microbiota from SLE patients and not seen with healthy controls [23]. This increase in Th17 was also linked to calcium/calmodulin-dependent proteinase kinase IV(CAMK4) and serine/threonine kinase, which is present at high levels in T cells in SLE patients [24]. As the diet and nutrient intakes of patients with SLE appear to play an important role in the disease development and complications [25], we investigated the dietary patterns and nutritional status of Omani females in the age range of 19-50 years based on case-control design.
2. Materials and Methods
2.1 Dietary patterns and nutrients Intake
The questionnaire was designed to provide data about the frequency of intake of a variety of food items as standardized portion sizes. Development of semi-quantitative food frequency questionnaire (SFFQ) went through four main steps. First step was the construction of food list based on other validated FFQ according to Block et al. [26] and previously used at SQU, which is based on the Omani food consumption patterns (Appendix 1). The Omani healthy plate is structured on 6-11 servings of grains and potatoes, 3-5 servings of vegetables, 2-4 servings of fruits, one serving of legumes and one serving of milk and dairy products [27]. Then portion sizes were defined for all food items. After that, the categories to assess the frequency of consumption were set from never to more than 6 times per day. At last, the FFQ was administered to a small group of patients and controls to determine the completeness of food list and to guide the generation of shorter food list for SFFQ by eliminating less commonly consumed food items from the questionnaire. Similar method was used by Dehghan et al. [28] to develop SFFQ based on local food types in United Arab Emirates and Kuwait. The average frequency of consumption in SFFQ was assessed by five categories (never, rarely, monthly, weekly and daily) that are based on the mid-point of the frequencies used in FFQ; for example, daily is equivalent to 4 servings per day.
Ethical approval for conducting the study was obtained from the ethics committee at the college of medicine and health sciences, Sultan Qaboos University (MERC# 1418). This part of the questionnaire was interviewer- administered for all cases to reduce the errors comes from weak understanding of the serving sizes and to clarify any other questions regarding the food types. For 95% of controls, the questionnaire was self-administered and a supplementary sheet was sent to explain the portion sizes for each food item and extra details regarding some food items (Appendix 2). Participants that were admixed or males were excluded from the study.
The semi-quantitative FFQ assessed the intake of 30 food items and the participants indicated their average intake for each food item over the last 3 months of a specified serving size. Nutrient intake was then calculated using computerized software program (Micro.diet V4, publisher Downlee System Limited) in collaboration with SQUH dietitians that multiply the reported frequency of each food item by the amount of nutrients in a serving of that food item. The nutrients analysis covered both macro and micronutrients including; protein, fat, carbohydrate, sugar, sodium, potassium, calcium, water-soluble vitamins (B6, B12, C) and fat-soluble vitamin (D). The frequency of consumption recorded in five categories (once a month, weekly, daily and rarely, never). For the analysis of the FFQ, survey answers were converted into numeric factors that represent the frequency of consumption per week. These numbers were then plugged in the food intake frequency template (Appendix 3) that is used to calculate the total intake of nutrients in all food items per week for each participant. After that, the average of total intake of nutrients of patients was compared with controls.
2.2.1 Dietary patterns: Principle component analysis (PCA) was carried to predict food groups as possible risk factors for SLE [6].
2.2.2 Nutrient Intakes: The frequency of each nutrient was calculated as described above and compared to the accepted reference used by SQUH, “Oman Food Based Dietary Guidelines” published by Ministry of Health in Cooperation with UNICEF and international standards. It is also coupled with the Adequate Intake (AI) and Recommended daily allowances (RDAs) international standards that both could be used as a reference of daily intake of macro and micronutrients [29]. The intake of macronutrients was compared between cases and controls using ANNOVA, multiple t tests.
3. Results
One hundred and twenty participants (60 cases and 60 controls) completed a data collection of their dietary intake using a semi-quantitative food frequency questionnaire (SFFQ). The PCA figure showed a trend of cases clustering with high intake of beef, oil and fruits (Percentage of variance of PC1 is 16.176 PC2 11.659) (Figure 1).
3.1 Nutrient intakes
The analysis of the intake of nutrients was based on the assessment of diet from the semi-quantitative FFQ (Table 1 and Table 2). Macronutrients analysis did not show any significant differences between cases and controls. Therefore, the levels of macronutrients will be compared to RDAs and AI to evaluate the results. The energy intake of cases (1505.88 Kcal/d) was lower than RDA (2200 Kcal/d) while proteins and fats intake were higher than RDA (Table 1). Carbohydrates intake was higher than the recommended AI (130 g/day) as reflected by the assessment of dietary intake from semi quantitative questionnaire. Micronutrient intakes by cases showed that the intake of calcium, magnesium, iron, selenium, iodine, vitamins B6, D and E was lower than RDA. Whereas phosphorous and vitamin B12 intake was higher than RDA (Table 2).
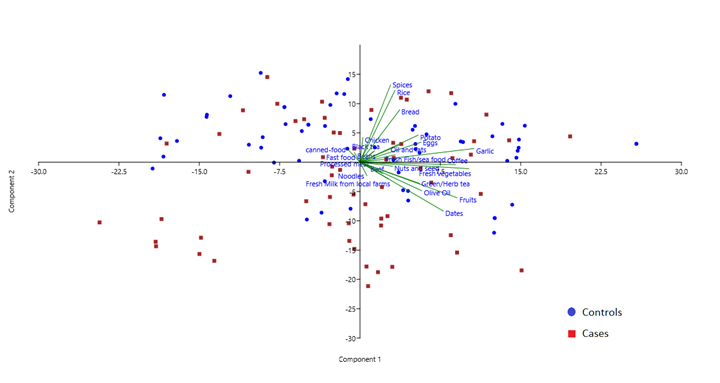
Figure 1: PCA of the intake of 30 food items by cases and controls.
|
Group Nutrient |
Cases N=60 |
Control N=60 |
t-test, P values |
|
Carbohydrates |
139.77 ± 63.03 |
147.227 ± 58.624 |
t= 0.6708, P=0.503673 |
|
Protein |
72.80 ± 35.93 |
70.742 ± 38.512 |
t= 0.3020, P=0.763158 |
|
Fats |
78.20 ± 38.17 |
76.769 ± 44.828 |
t=0.1886, P=0.850732 |
|
Energy |
1505.88 ± 662.91 |
1515.854 ± 717.375 |
t=0.07906, P=0.937120 |
Data are expressed as mean ± S.D; Recommended dietary allowances (RDA) for Caloric requirements (2200.00-2600.00 kcal/day), Protein requirements (46 g/day), Carbohydrate requirements (130 g/day), Fat (AI) (25 g/day)
Table 1: Average Daily Macronutrient Intakes of Study Subjects.
|
Group Micronutrient |
Cases (n=60) |
Controls (n=60) |
P value |
RDA |
|
Na (mg/d) |
1501.333 |
1391.223 |
0.265 |
1600 |
|
Ca mg/d |
437.6276 |
245.067 |
0.351 |
1000 |
|
Mg mg |
181.8647 |
186.072 |
0.746 |
320 |
|
P (mg/d) |
867.4423 |
807.3 |
0.118 |
700 |
|
Iron (mg/d) |
9.805529 |
9.428 |
0.251 |
18 |
|
Se (mg/d) |
18.39314 |
17.958 |
0.742 |
55 |
|
Iodine (mg /d) |
47.72675 |
48.104 |
0.650 |
150 |
|
VitB6 mg/d |
1.042937 |
1.049 |
0.597 |
1.3 |
|
VitB12 mg /d |
5.345708 |
3.933 |
0.006 |
2.4 |
|
VitC mg/d |
57.97084 |
52.172 |
0.153 |
75 |
|
VitD mg /d |
2.743525 |
1.926 |
0.006 |
15 |
|
VitE mg/d |
0.854426 |
0.895 |
0.843 |
15 |
AI for sodium is shown in bold
Table 2: Nutrients differences between cases and controls.
4. Discussion
SLE is a multifactorial disease affected by genetic, environmental and hormonal factors. Therefore it is hard to establish relationship between nutrition and SLE activity although there are evidence for the influence of diet quality in the development of clinical complications, such as low bone mineral density and cardiovascular diseases, in SLE cases [30]. Nutrients like selenium, calcium, ω-3 fatty acids, vitamins E, A and D reported to have beneficial effects on disease symptoms. On the other hand, high intake of fats, calories, protein, zinc and iron can worsen disease symptoms [31]. SFFQ found to be easier to administer and more reliable as reported by others [28]. Pre-specified serving sizes was incorporated in the SFFQ instead of giving the participants the opportunity to specify their portion sizes to make the calculations easier and more consistent. Although self-administration of the questionnaire to controls might have biased the results, but this was the only way to include participants from different backgrounds and educational levels instead of focusing on the university students and staff. Three dietary patterns of cases were determined; meat, fats and fruits. High meat dietary pattern was reported earlier in Japanese SLE females but did not have measurable association with SLE risk [6].
The adequate caloric intake for SLE cases is 1,200-1,400 kcal/day for men and 1,000-1,200 kcal/day for women [12, 33], which is lower than energy intake of the cases in our study that is 1505.88 Kcal/day. It is known that the progression of autoimmune diseases is altered by the restriction of calories in the diet [33]. Energy restriction prevent the reduced production of CD4+ and CD8+ T lymphocytes and increased Th1 cytokines (IL2 and IFN-g) in NZB/NZW mice [8]. In addition, Calories restriction can induce changes in the gut microbiota that was proven to benefit the immune system [33]. In humans, the risk of fetal loss in women with SLE and antiphospholipid syndrome was reduced when their diet included higher levels of polyunsaturated fatty acids (PUFA) [34].
In our study, the results showed high daily intake of proteins and fat by both cases and controls compared to RDA. Although there are no significant differences between the two groups but the average daily intake of proteins and fats by cases is more than controls. There are studies suggesting the association of proteins with complications of SLE diseases. In NZB/NZW mice, studies have revealed that moderate-protein diet-fed mice had a delay in autoimmunity and complication of SLE compared with normal-protein diet- fed mice. In addition, a restricted diet of phenylalanine and tyrosine amino acids was associated with beneficial effects on these mice [12]. Excessive protein intake lead to constant bone mineral loss in patients with juvenile SLE, whereas the consumption of 0.6 g/kg/day in a protein-restricted diet has enhanced the glomerular filtration rate in pre-dialytic chronic kidney disease of patients with systemic diseases [8].
Changes in the metabolism of lipoproteins and lipid profile in SLE patients is related to autoimmunity and inflammation in the disease. It was reported that both active and remission status of SLE, have higher levels of triglycerides (TG) and very low-density lipoprotein cholesterol (VLDL-C), which is associated with lower levels of high-density lipoprotein cholesterol (HDL-C). The enzymatic activity of lipoprotein lipase is reduced in SLE leading to reduced catabolism of TG-rich lipoproteins by either the presence of anti-lipoprotein lipase antibodies (anti-LPL) or tumor necrosis factor-α (TNF- α) action [8]. This suggest that high consumption of fat seen in this study can elaborate the problems associated with lipid metabolism in SLE cases. Balanced diet is important to avoid complications associated with SLE such as cardiovascular diseases, diabetes, obesity, metabolic syndrome and dyslipidemia [8, 12].
The intake of vitamin B12 is usually associated with the consumption of animal origin foods that are the only dietary sources of this vitamin [35]. Although there was no measurement of vitamin B12 concentration in plasma, participants in our study had high levels of vitamin B12 as reflected by the data analysis of dietary intake in the SFFQ unlike other studies showing low vitamin B12 in cases due to low intake of meat and beans [35]. This is due to the higher intake of food from animal origin by our study group. The recommended daily intake of vitamin B12 is 2-3 mg that balanced diet could easily provide [36, 37]. Some studies discussed the fact that the recommended value (2-3 mg/d) is not sufficient to maintain genomic stability recommending to increase the daily intake to 7 mg/day. This daily intake is probably sufficient for a plasma level of 400 pg/ml [38] which is known to decrease micronucleus formation in lymphocytes [36, 39].
Deficiency or low levels of vitamin D was linked with increased susceptibility of SLE and increased activity-SLEDAI [40]. Nutrient analysis of cases participating in this study showed low levels of vitamin D of 2.74 mg/d compared to RDA of 15 mg/d. The steroid hormone vitamin D have essential role in immune system homeostasis and mineral metabolism [41, 42]. The active form of vitamin D (calcitriol) regulate T and B cell responses and boost the innate immune response. In this sense, low levels of vitamin D in the body is a possible trigger for SLE and other autoimmune disease [43, 44]. Low levels of vitamin D is seen in cases and controls in this study as this is one of the common deficiencies in Oman especially in females [45]. This could be due to the low intake of milk and dairy products as part of the dietary habits in the country as we found 72% of the total participants in this study are consuming less than 2 portions of milk per day. The general practice for SLE patients at SQUH is supplementation with vitamin D and monitoring its levels between routine visits. Supplementation with vitamin D have significant beneficial effects on the maturation and activation of dendritic cells [46] in addition to bone mineral density when the plasma level reaches >36.8 ng/l [47].
The intake of vitamin E by cases (0.854 mg/d) was very low compared to RDA that can worsen disease status in these cases since it is known for the ability to decrease the levels of inflammatory cytokines, IL-2, IL-4 and TNF-a when combined with omega3 PUFA from fish oil [8]. The study showed that the average intakes of calcium, iron, selenium, magnesium, Iodine, vitamins B6, D and E for cases are lower than RDA. Selenium is an important antioxidant and critical component in a number of antioxidant enzymes and immune functions. In autoimmune diseases, selenium serum levels were lower than healthy controls is possibly associated with the initiation of inflammation and auto immunity [48]. Similar observations for calcium and iron were reported earlier in patients compared to healthy controls [35].
5. Conclusion
The dietary habits of the Omani females in this study showed high protein and fat intake with low levels of important micronutrients like vitamin C, vitamin D, calcium and selenium, which may be associated with SLE complications in the long term. Replication of the questionnaire recruiting more number of SLE cases and healthy controls will help in confirming the findings. This will help in suggesting healthy dietary plan for patients aiming for better management of the disease and possibly prolongs the remission status of patients.
Acknowledgements
Special acknowledgment to the Aliya Al-Moqbali for her help in the questionnaire conduction.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Malgorzata J Podolska, Mona HC Biermann, Christian Maueröder, et al. Inflammatory etiopathogenesis of systemic lupus erythematosus: An update. Journal of Inflammation Research 8 (2015): 161-171.
- Kamen DL. Environmental influences on systemic lupus erythematosus expression. Rheumatic Disease Clinics of North America W.B. Saunders 40 (2014): 401-412.
- Hu FB. Dietary pattern analysis: A new direction in nutritional epidemiology. Current Opinion in Lipidology 13 (2002): 3-9.
- Minami Y, Sasaki T, Komatsu S, et al. Female systemic lupus erythematosus in miyagi prefecture, japan: A case-control study of dietary and reproductive factors. Tohoku Journal of Experimental Medicine 169 (1993): 245-252.
- Nagata C, Fujita S, Iwata H, et al. Systemic lupus erythematosus: a case-control epidemiologic study in Japan. International Journal of Dermatology 34 (1995): 333-337.
- Kiyohara C. Rythematosus in a Japanese population: The Kyushu Sapporo SLE (KYSS) StudyDietary patterns and the risk of systemic lupus e. International Medical Journal 22 (2015): 110-115.
- Dunnage J, Rahman A. Lupus and Healthy Eating. UK (2019).
- Klack K, Bonfa E, Borba Neto EF. Diet and nutritional aspects in systemic lupus erythematosus. Revista brasileira de reumatologia 52 (2012): 384-408.
- Dina S Fettouh, Dalia S Saif, Saga F El Gazzar, et al. Study the relationship between vitamin A deficiency, T helper 17, regulatory T cells, and disease activity in patients with systemic lupus erythematosus. Egyptian Rheumatology and Rehabilitation 46 (2019): 244-250.
- Borja Sánchez, Arancha Hevia, Sonia González, et al. Interaction of intestinal microorganisms with the human host in the framework of autoimmune diseases. Frontiers in Immunology 6 (2015): 594.
- Handono K, Firdausi SN, Pratama MZ, et al. Vitamin A improve Th17 and Treg regulation in systemic lupus erythematosus. Clinical Rheumatology. Springer London 35 (2016): 631-638.
- Maria-Magdalena Constantin, Iuliana Elena Nita, Rodica Olteanu, et al. Significance and impact of dietary factors on systemic lupus erythematosus pathogenesis. Experimental and Therapeutic Medicine. Spandidos Publications 17 (2019): 1085-1090.
- Hechtman L. Clinical Naturopathic Medicine. Luisa Cecotti (2014).
- Yuko Minami, Yasuhiko Hirabayashi, Chisato Nagata, et al. Intakes of vitamin B6 and dietary fiber and clinical course of systemic lupus erythematosus: A prospective study of Japanese female patients. Journal of Epidemiology 21 (2011): 246-254.
- Abaza NM, El-Mallah RM, Shaaban A, et al. Vitamin D deficiency in Egyptian systemic lupus erythematosus patients: How prevalent and does it impact disease activity?. Integrative Medicine Insights Integr Med Insights 11 (2016): 27-33.
- Ritterhouse LL, Lu R, Shah HB, et al. Vitamin D deficiency in a multiethnic healthy control cohort and altered immune response in vitamin D deficient European-American healthy controls. PLoS ONE 9 (2014): e94500.
- Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. American Association for the Advancement of Science 334 (2011): 105-108.
- Inés Martínez, James M Lattimer, Kelcie L Hubach, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME Journal 7 (2013): 269-280.
- Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336 (2012): 489-493.
- Tanya Yatsunenko, Federico E. Rey, Mark J. Manary, et al. Human gut microbiome viewed across age and geography. Nature 486 (2012): 222-227.
- Manichanh C, Borruel N, Casellas F, et al. The gut micro- biota in IBD. Nat Rev Gastroenterol Hepatol 9 (2012): 599-608.
- Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2 (2013): e01202.
- Patricia López, Banesa de Paz, Javier Rodríguez-Carrio, et al. Th17 responses and natural IgM antibodies are related to gut microbiota composition in systemic lupus erythematosus patients. Scientific reports. Nature Publishing Group 6 (2016): 24072.
- Juang YT, Wang Y, Solomou EE, et al. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. Journal of Clinical Investigation 115 (2005): 996-1005.
- Oeser A, Chung CP, Asanuma Y, et al. Obesity is an independent contributor to functional capacity and inflammation in systemic lupus erythematosus. Arthritis and rheumatism 52 (2005): 3651-3659.
- Block G, Woods M, Potosky A, et al. Validation of a self-administered diet history questionnaire using multiple diet records. Journal of Clinical Epidemiology 43 (1990): 1327-1335.
- Ministry of Health Sultanate of Oman. The Omani Guide to Healthy Eating. Muscat (2009).
- Dehghan M, Al Hamad N, Yusufali A, et al. Development of a semi-quantitative food frequency questionnaire for use in United Arab Emirates and Kuwait based on local foods. Nutrition Journal 4 (2005): 18.
- Gibson RS. Principles of Nutritional Assesment. second. New York: Oxford University Press (2005).
- Shah M, Adams-Huet B, Kavanaugh A, et al. Nutrient intake and diet quality in patients with systemic lupus erythematosus on a culturally sensitive cholesterol lowering dietary program. J Rheumatology 31 (2004): 71-75.
- Brown AC. Lupus erythematosus and nutrition: A review of the literature. Journal of Renal Nutrition. W.B. Saunders 10 (2000): 170-183.
- National Institutes of health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. The American Journal of Clinical Nutrition. Oxford University Press (OUP) 68 (1998): 899-917.
- Vieira S, Pagovich O, Kriegel M. Diet, microbiota and autoimmune diseases. Lupus 23 (2014): 518-526.
- Reifen R, Amital H, Blank M, et al. Linseed oil suppresses the anti-beta-2-glycoprotein-I in experimental antiphospholipid syndrome. Journal of Autoimmunity 15 (2000): 381-385.
- Borges MC, dos Santos Fde M, Telles RW, et al. Nutritional status and food intake in patients with systemic lupus erythematosus. Nutrition 28 (2012): 1098-1103.
- Fenech MF, Dreosti IE, Rinaldi JR. Folate, vitamin B12, homocysteine status and chromosome damage rate in lymphocytes of older men. Carcinogenesis 18 (1997): 1329-1336.
- Andrès E, Serraj K, Zhu J, et al. The pathophysiology of elevated vitamin b12 in clinical practice. QJM 106 (2013): 505-515.
- Solomon LR. Disorders of cobalamin (Vitamin B12) metabolism: Emerging concepts in pathophysiology, diagnosis and treatment. Blood Reviews 21 (2007): 113-130.
- Fenech M, Aitken C, Rinaldi J. Folate, vitamin B12, homocysteine status and DNA damage in young Australian adults. Carcinogenesis 19 (1998): 1163-1171.
- Peggy W Wu, Elisa Y Rhew, Alan R Dyer, et al. 25-Hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Care and Research 61 (2009): 1387-1395.
- Kamada N, Seo SU, Chen GY, et al. Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews Immunology 13 (2013): 321-335.
- Mu Q, Zhang H, Luo XM. SLE: Another autoimmune disorder influenced by microbes and diet?. Frontiers in Immunology 6 (2015): 608.
- Ritterhouse LL, Crowe SR, Niewold TB, et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann Rheum Dis 70 (2011): 1569-1574.
- Antico A, Tampoia M, Tozzoli R, et al. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev 12 (2012): 127-136.
- Al-Kindi MK. Vitamin D Status in Healthy Omani Women of Childbearing Age: Study of female staff at the Royal Hospital, Muscat, Oman. Sultan Qaboos University medical journal 11 (2011): 56-61.
- Ben-Zvi I, Aranow C, Mackay M, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS ONE 5 (2010): 9193.
- Kamen DL, Cooper GS, Bouali H, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmunity Reviews Elsevier 5 (2006): 114-117.
- Sahebari M, Rezaieyazdi Z, Khodashahi M. Selenium and Autoimmune Diseases: A Review Article. Current Rheumatology Reviews 15 (2018): 123-134.
