Cerebral Air Embolism: A Non-Thrombotic Cause of Acute Stroke
Article Information
Martina Squitieri MD1, Anna Poggesi MD, PhD1,4,5, Andrea Cecchi MD2, Gabriella Di Lascio MD2, Davide Gadda MD3, Ivano Lombardo MD3, Francesca Pescini MD, PhD4*
1NEUROFARBA Department, University of Florence, Italy
2SODC Intensive Care of Trauma and Severe Organ Failure, Hyperbaric and Diving Medicine section, Neuromusculoskeletal Department, AOU Careggi, Florence, Italy
3Neuroradiology Unit, Department of Services, AOU Careggi, Florence, Italy
4Stroke Unit, Emergency Department, AOU Careggi, Florence, Italy
5IRCCS Don Carlo Gnocchi, Florence, Italy
*Corresponding Author: Dr. Francesca Pescini, Stroke Unit, Emergency Department, AOU Careggi, Largo Brambilla 3, 50134, Florence, Italy
Received: 27 August 2020; Accepted: 21 September 2020; Published: 12 November 2020
Citation: Martina Squitieri, Anna Poggesi, Andrea Cecchi, Gabriella Di Lascio, Davide Gadda, Ivano Lombardo, Francesca Pescini. Cerebral Air Embolism: A Non-Thrombotic Cause of Acute Stroke. Archives of Clinical and Medical Case Reports 4 (2020): 1071-1077.
View / Download Pdf Share at FacebookAbstract
Background: Embolic strokes are due to occlusion of brain arteries mainly by clots, less frequently by non-thrombotic material. Cerebral air embolism (CAE) may occur as complication of medical procedures, although non-iatrogenic sources have been reported. It is a life-threating emergency with poor prognosis both in terms of mortality and disability. Hyperbaric oxygen therapy (HOT) is the gold standard treatment.
Methods: We report a series of 4 in-hospital patients with CAE for whom stroke neurologists were activated for acute management. Three patients developed neurological disturbances soon after central venous catheter manipulation, one patient, affected by chronic obstructive pulmonary disease, after prolonged coughing with respiratory distress.
Results: All patients experienced seizures, loss of consciousness and bilateral neurological signs. Acute brain computed tomography (CT) revealed intracranial air bubbles. One patient underwent HOT 3 hours after symptoms onset and fully recovered, for the others HOT was contraindicated: one died and 2 presented severe neurological sequelae.
Conclusions: Physicians involved in acute stroke management should be aware of CAE and promptly recognize it, especially for in-hospital strokes, and manage this condition together with anesthetists and hyperbaric physicians.
Keywords
Cerebral air embolism; Embolic stroke; Gas embolism; Non-thrombotic stroke
Article Details
1. Introduction
Embolic strokes are most commonly due to thrombi consisting in red blood cells, platelets and fibrin. However, emboli may have a different composition including air, fat, calcium, tumor cells, infective agents or substances used in medical procedures. Cerebral air embolism (CAE) is a rare but serious complication of several procedures, such as venous catheterization, neurosurgery, open heart surgery, thoracotomy, pneumoradiologic procedures, arterial angiography and endoscopy. More rarely, CAE is due to barotrauma, cranial or thoracic trauma. The incidence is 2.65/100.000 hospitalizations/year, but it is probably underestimated since many patients are undiagnosed or unreported [1]. Once in the bloodstream, air emboli cause cerebral edema and/or infarction by mechanically blocking small vessels and also eliciting an inflammatory response that results in endothelial damage [2]. Brain lesions are multifocal, which is consistent with multiple neurological deficits and severe pictures. CAE is a life treating condition, which requires an immediate management. Hyperbaric oxygen therapy (HOT) is the gold standard treatment to be performed within the first hours [1].
We report a series of four in-hospital patients affected by CAE for whom stroke neurologists were activated for the acute management.
2. Case Series
2.1 Patient 1
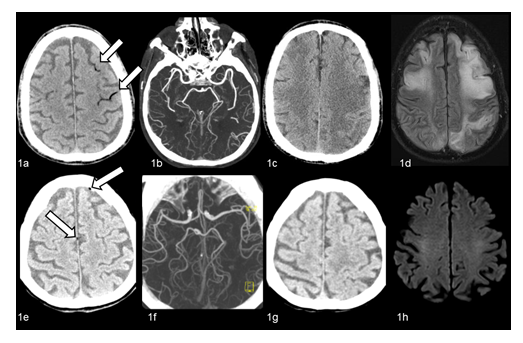
A 71-year-old man went to coronary artery bypass grafting for critical stenosis in the left main trunk. After twenty days, during manipulation of central venous catheter (CVC) (right jugular), sudden loss of consciousness with flexion withdrawal from pain occurred (Glasgow Coma Scale-GCS [3]=6/15). Twenty minutes later, brain CT revealed multiple hypodensities in the left parietal and frontal lobes sulci (Figure 1a); no arterial occlusion was found on CT angiography (CTA) (Figure 1b). Clinical picture rapidly worsened: respiratory insufficiency, severe hypotension and convulsive epileptic status occurred and the patient was intubated. Although CAE was suspected, HOT was not applied for the critical conditions. The patient developed an unresponsive wakefulness syndrome. Patent foramen ovale (PFO) was not detected with transesophageal echocardiography. Follow-up brain CT (Figure 1c) and MRI (FLAIR sequence) (Figure 1d) showed multiple bilateral fronto-parietal ischemic lesions.
2.2 Patient 2
A 84-year-old woman underwent cardio-surgery for aortic valve replacement. Twelve days later, right jugular CVC was removed and she presented abrupt onset of right leg clonic jerks and right deviation of head and eyes. She was aphasic with right arm plegia, paresis of the others limbs, right hemianopsia, hemianesthesia and facial palsy (National Institutes of Health Stroke Scale–NIHSS [4]=32/42, GCS [3]=9/15), and bilateral Babinski sign. Brain CT performed 20 minutes later revealed multiple hypodensities in the right frontal lobe sulci, both cavernous sinus and right jugular vein. CTA did not show vessels occlusions. CAE was hypothesized, but HOT was not applied since after contrast media administration, the patient developed acute hypercapnic respiratory failure requiring non-invasive ventilation. In the following days, neurological deficits partially improved. At discharge right arm and both legs paresis were still present. Brain CT follow-up showed multiple bilateral fronto-parietal ischemic lesions.
2.3 Patient 3
A 71-year-old male underwent a coronary angiography for hearth failure and myocardial hypokinesia. He had end-stage renal disease, chronic obstructive pulmonary disease and during childhood experienced pulmonary lobectomy for a cystic lesion. On admission, pneumonia was diagnosed. On day 2, after prolonged coughing and subsequent acute respiratory distress, a radial artery access was placed. Soon after, the patient suddenly became unresponsive (GCS [3]=7/15) with left deviation of head and eyes. Thirty minutes later, brain CT revealed multiple hypodensities in right temporo-occipital sulci. At CTA performed 40 minutes later vessels occlusions was not detected and the hypodensities were not visible. CAE was diagnosed. HOT was contraindicated because of pneumothorax and pneumomediastinum detected on chest CT, severe respiratory failure and the occurrence of uncontrolled clonic seizures. Death occurred 3 days later for multiple organ failure.
2.4 Patient 4
A 82-year-old female underwent mitral valve plastic surgery and closure of oval foramen through right minithoracotomy. Five days later, soon after CVC removal (right jugular), she became unresponsive. She was awake with right gaze deviation, aphasic, tetra-paretic, and with left hemianopia (NIHSS [4]=27/42). Echocardiogram revealed air in the right cameras. CAE was hypothesized. On brain CT performed 30 minutes later, air was found inside left frontal subarachnoid space and along the lower edge of falx cerebri (Figure 1e). No major vessels occlusion was found on CTA (Figure 1f). HOT was started 3 hours after the symptoms onset. Hyperbaric session was scheduled according to table 6 US Navy, but given to initial clinical recovery, table 5 US navy was adopted [4, 5]. At the end of treatment, she was tired, poorly collaborating, febrile and a generalized seizure occurred. Brain CT showed intracranial air reabsorption (Figure 1g). The day after, the neurological picture progressively improved. Brain CT (48 hours) and MRI (10 days) (Figure 1h) did not reveal new lesions. At discharge (11 days later) no neurological deficits were present.
Figure 1: 1a-1d: Patient 1. On unenhanced brain CT performed 20 minutes after sudden loss of consciousness, air bubbles are visible inside the sulci of left parietal and frontal lobes (Figure 1a, arrows). No evidence of intracranial arterial occlusions on CT angiography (Figure 1b). Follow-up CT (3 days later, Figure 1c) and MRI (FLAIR sequences) (12 days later, Figure 1d) show multiple bilateral fronto-parietal ischemic lesions. 1e-1h: Patient 4. On unenhanced CT performed 30 minutes after sudden symptoms, minimal air bubbles are visible inside left frontal subarachnoid space and along the lower edge of the falx cerebri (Figure 1e, arrows). No evidence of intracranial arterial occlusions on CT angiography (Figure 1f). Unenhanced CT performed after HOT shows intracranial air resorption (Figure 1g), with no evidence of new brain lesions. On diffusion-weighted (DWI) MRI performed 10 days later (Figure 1h) no evidence of brain areas of diffusion restriction consistent with recent ischemic lesions.
3. Discussion
Non-thrombotic causes of embolic stroke are rare; however, the increasing availability of interventional and intravascular procedures may lead to increased incidence of these stroke types, mainly CAE. Anesthetists and physicians of intensive care units face this severe complication as a consequence of high-risk interventions (cardiovascular surgery using extracorporeal circulation or neurosurgery), but it may occur also in less intensive settings. Indeed, the vast majority of iatrogenic CAE are due to CVC manipulation [6]. Neurological sequelae or death characterize the outcome of CAE in a high percentage of cases [1, 7]. Neurologists and stroke physicians should be aware of this condition in order to promptly recognize and manage it together with anesthetists and hyperbaric physicians.
CAE diagnosis may be difficult, and a high index of suspicion is needed in the clinical setting where air may reach the cerebral vasculature [6]. The symptoms mimic acute thrombotic stroke, although they are usually more severe, with multifocal deficits, consciousness disturbance and epileptic seizures often leading to epileptic status. Prompt brain CT may support diagnosis: intracranial air can be detected within 30 minutes after symptoms onset, rarely up to 38 hours. It depends on the amount of air, bubble's size, whether venous or arterial involvement [8]. Air emboli on CT are small rounded or curvilinear hypodensities close to cortical sulci, intraparenchymal or in intracranial veins or venous sinus.
CAE can occur as venous or arterial embolism. Once air enters the venous circulation, a retrograde ascension to cerebral veins, especially in upright position, can take place, thanks to its low specific gravity with consequent venous outflow impairment [9] (probably it occurred in patient 2). Otherwise, air enters directly into the arteries (i.e., angiography) or reaches arterial circulation from the venous one through ventricular septal defect, PFO, pulmonary arteriovenous malformations or pulmonary microcirculation [10]. We did not found a PFO in patient 1, while patient 4, who had air in both right and left cameras, PFO had been closed before CAE. In both patients, air could have entered in arteries through a temporarily open or not completely closed PFO. Indeed, when large air volume enters the pulmonary arteries, it could cause air-lock leading to pulmonary hypertension and right heart strain. This increased pressure can temporarily open a PFO and allow paradoxical air embolism [5, 9]. Otherwise, air could reach arteries through pulmonary microcirculation. This may occur when the pulmonary filter is overwhelmed by a large amount of air that cannot be absorbed and may pass through the pulmonary circulation across arteriovenous shunts thus entering the left side of the heart. In this situation, intrapulmonary arteriovenous anastomoses (IPAVA) are recruited and the passage of air bubbles is allowed [11]. In patient 3 the increase in the intrapulmonary pressure due to cough may have caused rupture of the alveoli and damage of vessels wall allowing the passage of air into the bronchial veins and then in the left ventricle. Therefore, underlying pulmonary vulnerability can be considered a risk factor for CAE in non-iatrogenic situations [7, 8, 12].
Treatment of CAE includes both supportive treatment and HOT, but first the entry of air must be reduced [13]. The patient should be placed in Trendelenburg’s position with a left lateral head down tilt to prevent the movement of air into the right ventricular outflow tract and 100% oxygen should be administered to encourage resorption from the vasculature [5, 7]. Once stabilized, urgent HOT may decrease the size and effects of the emboli. HOT helps to reduce bubbles size, facilitates diffusion of oxygen in ischemic tissues and inhibits inflammation through the inhibition of neutrophil attachment to blood vessels. The outcome depends on the time to HOT. Indeed, according to some authors, HOT is associated with favorable prognosis if started within 6 hours from the symptoms onset [6]. However, the critical time interval after injury is uncertain and has been variably reported to be 3-48 hours. Hyperbaric treatments are recommended until there is improvement, generally no more than one or two, occasionally up to ten [14]. Patient 4 underwent HOT at 3 hours from symptoms onset and she was the only patient with good outcome. Although no trial or prospective studies have been conducted on CAE patients, reviews of published cases reveal better outcomes with HOT compared to no-recompression treatment. HOT is indicated in guidelines for patients with gas embolism and neurological manifestations [14, 15]. Serious adverse effects rarely occur during treatment resulting from oxygen toxicity, such as seizures, congestive heart failure exacerbation, pulmonary edema, and retinal changes.
The most serious contraindication to HOT is untreated pneumothorax, which would worsen under chamber pressure. In the Italian guidelines absolute contraindications are also a ratio between the partial pressure of oxygen and fraction of inspired oxygen (PaO2/FiO2) <200, hypertensive pneumothorax and generalized epileptic status [16]. Relative contraindications include obstructive lung disease, asymptomatic pulmonary blebs or bullae, poorly controlled seizure disorder, and congestive heart failure. However, these relative contraindications should not deter clinicians from using HOT in patients with severe neurological deficits. If CAE is suspected, hyperbaric physicians should be quickly activated in order to rapidly discover and manage contraindications and start HOT.
References
- Bessereau J, Genotelle N, Chabbaut C, et al. Long-term outcome of iatrogenic gas embolism. Intensive Care Med 36 (2010): 1180-1187.
- Hulst RA, Klein J, Lachmann B. Gas embolism: pathophysiology and treatment. Clin Physiol Funct Imaging 23 (2003): 237-246.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet (London, England) 2 (1974): 81-84.
- Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother 60 (2014): 61.
- Naval Sea Systems Command. US Navy Diving Manual Rev. 7. J Trauma Stress 2 (2016): 991.
- Blanc P, Boussuges A, Henriette K, et al. Iatrogenic cerebral air embolism: importance of an early hyperbaric oxygenation. Intensive Care Med 28 (2002): 559-563.
- Pinho J, Amorim JM, Araujo JM, et al. Cerebral gas embolism associated with central venous catheter: Systematic review. J Neurol Sci 362 (2016): 160-164.
- Weiss KL, Macura KJ, Ahmed A. Cerebral air embolism: acute imaging. J Stroke Cerebrovasc Dis 7 (1998): 222-226.
- Schlimp CJ, Loimer T, Rieger M, et al. Pathophysiological mechanism and immediate treatment of retrograde cerebral venous air embolism. Intensive care medicine 32 (2006): 945.
- Khan H, Zaidi A. Paradoxical air embolism following central venous catheter removal. BMJ Case Rep (2013): 1-3.
- Lovering AT, Duke JW, Elliott JE. Intrapulmonary arteriovenous anastomoses in humans - response to exercise and the environment. J Physiol 593 (2015): 507-520.
- Gudmundsdottir JF, Geirsson A, Hannesson P, et al. Major ischaemic stroke caused by an air embolism from a ruptured giant pulmonary bulla. BMJ Case Rep (2015): 2014-2016.
- Shaikh N, Ummunisa F. Acute management of vascular air embolism. J Emerg Trauma Shock 2 (2009): 180-185.
- Moon RE. Hyperbaric treatment of air or gas embolism: current recommendations. Undersea Hyperb Med 46 (2019): 673-683.
- Mathieu D, Marroni A. Tenth European Consensus Conference on Hyperbaric Medicine: recommendations for accepted and Tenth European Consensus Conference on Hyperbaric Medicine: 47 (2017): 24-32.
- Iperbarica Sidmse. Ossigenoterapia Iperbarica (Oti): Controindicazioni Ed Effetti Collaterali (2018): 1-18.