Carriage Profile of Transient Oral Bacteria among Dar es Salaam Hypertensive Patients and its Association to Hypertension
Article Information
Boaz Cairo, George Msema Bwire*, Kennedy Daniel Mwambete
Department of Pharmaceutical Microbiology, School of Pharmacy, Muhimbili University of Health and Allied Sciences, Box 65001, Dar es Salaam, Tanzania
*Corresponding Author: George Msema Bwire, Department of Pharmaceutical Microbiology, School of Pharmacy, Muhimbili University of Health and Allied Sciences, Box 65001, Dar es Salaam, Tanzania
Received: 03 July 2019; Accepted: 05 August 2019; Published: 07 August 2019
Citation: Boaz Cairo, George Msema Bwire, Kennedy Daniel Mwambete. Carriage Profile of Transient Oral Bacteria among Dar es Salaam Hypertensive Patients and its Association to Hypertension. Archives of Microbiology & Immunology 3 (2019): 094-101.
View / Download Pdf Share at FacebookAbstract
Background: The causes of hypertension can be either reversible and/or irreversible. Oral infections caused by transient normal flora, Porphyromonas gingivalis in particular is among the reversible factors which can directly or indirectly influence hypertension. Therefore, the study was conducted to determine the oral bacterial profile and establish an association between P. gingivalis carriage and hypertension.
Methods: A hospital based cross sectional study was conducted between January and July 2018 at Muhimbili National Hospital, Dar es Salaam. Oral swabs were collected and cultured in the appropriate media for isolation of bacteria. Bacterial identification was done using cultural properties and series of biochemical tests. Cramer’s V test analyzed categorical variables while Pearson correlation analyzed continous variables. Logistic regression was used in determination of Odds Ratio (OR). P value less than 0.05 was considered statistically significant.
Results: In 120 hypertensive patients (HTP) the most isolated bacterial species were Streptococcus pyogenes, Streptococcus agalactiae and Staphylococcus aureus by 20.6%, 18.8%and 7.1% respectively while for 50 nonhypertensive patients (NHTP) were 9.4%, 8.2% and 2.9% for S. pyogenes, S. agalactiae and S. aureus respectively. The prevalence of P. gingivalis was 10.6% in HTP and 1.2% in NHTP (p < 0.05). There was no significant association between harbouring P. gingivalis and hypertension (OR = 4.2; 95%CI = 0.944 -18.993; p = 0.130).
Conclusions: Hypertensive patients exhibit high oral carriage of transient bacteria especially P. gingivalis as compared to non-hypertensive individuals. However, there was no association between P. gingivalis carria
Keywords
Bacterial profile; Hypertension; Muhimbili National Hospital; Porphyromonas gingivalis
Article Details
Abbreviations
CRF: Case Report Form; CI: Confidence Interval; HP: Hypertension; HTP: Hypertensive Patients; MUHAS: Muhimbili University of Health and Allied Sciences; NHTP: Non-Hypertensive Patients
Introduction
Hypertension (HP) is a chronic disease characterized by raising blood pressure above normal range, which is above 120 mm Hg of systolic blood pressure (SBP) and greater than 80 mm Hg of diastolic blood pressure (DBP) [1]. The disease is one of the most prevalent and potential contributors to cardiovascular problems, and most people die of it or the associated diseases and complications. Two main types of HP are known so far namely primary or essential hypertension (EHP) and secondary hypertension (SHP). The former is idiopathic HP with no identifiable specific cause, since there are many associated factors that result into EHP, that include environmental factors, foods taken such as those with high content of tyramines, saturated fats and cholesterols, tobacco smoking, lack of exercise, chronic caffeine consumption, just mentioning a few [2]. Secondary hypertension is caused by underlying identifiable and correctable causes, such as conditions affecting kidneys, arteries, pregnancy, heart or endocrine system [3,4]. Basing on the risk factors of HP, the causes can be modifiable such as obesity, chronic alcoholism, lack of exercises, excessive sodium salt intake, diseases conditions such as liver diseases, diabetes mellitus and infections; while non-modifiable risk factors include age, gender, family history and race [5,6].
Microbial community of the human oral cavity is colonized by myriad of bacterial species [7], which sturdily affects the development and maintenance of immune homeostasis, operates as a barricade against pathogenic microbes invasion, and provides the host with nutritional elements [8]. However, oral infections have been attributing to the increase in HP, because several periodontal bacteria like Porphyromonas gingivalis, Streptococcus agalactiae Streptococcus pyogenes and Staphylococcus aureus are implicated in infections like periodontitis, dental carries and gingivitis [9,10]. The bacteria have been also detected in circulatory system and in aortic tissues, whereby experimental studies demonstrate that they act as pro-atherogenic stimuli [8]. Of these bacteria, P. gingivalis is more prevalent than others [9,10]. Such bacteria can disseminate to other parts of the human body causing damages to the blood vessels or producing biofilms, endotoxins and produce adhesive molecules like fimbriae and capsular polysaccharide that contribute to atherosclerosis and leading to HP [11].
To date, no study has been conducted at Muhimbili National Hospital (MNH) to determine oral bacterial profile and association between P.gingivalis infections with HP among hypertensive patients.
Materials and Methods
Study design
This was a cross sectional study involving collection of mouth swab specimens from conseted hypertensive patients and non hypertensive individuals at MNH between January and July 2018. The specimens were then sent to Central Pathology Laboratory (CPL) at MNH for bacteria isolation, and subsequently identification through colony characteristics and biochemical tests [12].
Study area and population
The study was conducted at MNH, Tanzania which is a tertiary referral hospital situated in the largest commercial city of Dar es Salaam of over 5 million residents, though receives patients from all over the country of about 60 million people [13]. Both hypertensive and non-hypertensive patients who sought medical care services at the Out Patients Department (OPD) were recruited in the study.
Sampling and sample size determination
The random sampling technique was employed to recruit hypertensive and non-hypertensive patients attending OPD at MNH. Relevant clinical and demographic information were collected through questionnaire, which also assisted in identification of appropriate subjects/patients as per study’s objectives (patients with suspected oral infections and/or hypertension). A sample size of 170 patients (120 hypertensive vs 50 non-hypertensive) was determined based on previous study by Fleming et al., [14].
Inclusion and exclusion criteria
Only patients over 31 years old, who were hypertensive, having generalized to severe, chronic periodontitis or tooth loss, and inflammation of the gum, were recruited in the study [15,16]. Pregnancy, smoking, systemic diseases that affect the gum or interfere with examination, consumption of antibiotics and corticosteroids during the past three months and clinical history of periodontal diseases in the past six months were used as exclusion criteria [17].
Samples collection procedures
Following consents from each patient, demographic and clinical information were collected through structured questionnaire as shortly described. Then sub-gingival specimens/swabs were collected using specialized tools and saliva bio-oral swab (SOS) assisted by competent health personnel. The specimens were then subjected onto reducing transport fluid (Stuart transport media) for easy transportation and enabling the bacteria to remain in their growth phase as that was observed once the samples were obtained from the patients, while waiting for aerobic and anaerobic procedures to take place.
Laboratory investigations
Laboratory culture, isolation and identification of bacteria species were performed as described by Japoni et al., [17].
Briefly; During laboratory work, the bacterial cultures were subjected to aerobic and anaerobic conditions. For anaerobic conditions Blood agar (BA) media were supplied with 5µg/mL of hemin and 1µg /mL of vitamin k, 7% of sheep blood and 3% of Tripticase soy broth (Sigma Chemical Co.) For aerobic growth, BA and Chocolate agar (CA) plates were used. Then the samples were incubated for 24 hours. For anaerobic conditions, the BA and CA plates were incubated in candle jar, with a wet paper placed strategically to control level of carbon-dioxide (CO2).Then the plates were re-incubated at 7% CO2 for 72 hours. The resultant bacterial colonies were identified by Gram staining followed by a series of biochemical tests.
Data Management and Analysis
Patients information ware collected in the patients’ files and documented in the case report form (CRF). Information collected in the were entered in the Microsoft excel sheet and then exported to statistical package for social science (SPSS version 23 software, Chicago Inc.,USA) for coding and analysis. The descriptive statistics was done using Cramer’s V for categorical variable and Pearson correlation for numerical variable. Logistic regression was used in determination of Odd ratios. Whereas linear regression was used to control continuous cofounding factors. P value was considered significant when read less than 0.05.
Ethical considerations
Following approval of this study by the Muhimbili University of Health and Allied Sciences, Ethical Committees and permission to recruit participants and laboratory investigation of samples was obtained from MNH authority, each patient was clearly informed on the objectives of the study prior to consenting verbally and in-writing. Patients were also informed that laboratory findings would be used for academic purpose and improvement of health care system. However, the results were also made available upon medical request for potential medical intervention. Names and other personal details were not disclosed for confidentiality purpose; thus, most information was coded and entered into the computer for statistical analysis.
Results
Demographics and factors attributable to oral problems in the study population
A total of 170 patients were recruited in this study, of those 98, 58% were males. Majority of participants had age above 50 years 87 (51.2%). Education levels of the participants ranged from informal education to tertiary/college level. Regarding habits of brushing their teeth, majority (67%) brushed their teeth using conventional toothpastes. About 92% (n=157) had at least lost one tooth as consequence of dental problems, while 98 (57.6%) patients had gingivitis. Slightly more than half (n=88; 52%) of the participants brushed their teeth thrice per day as shown on (Table1).
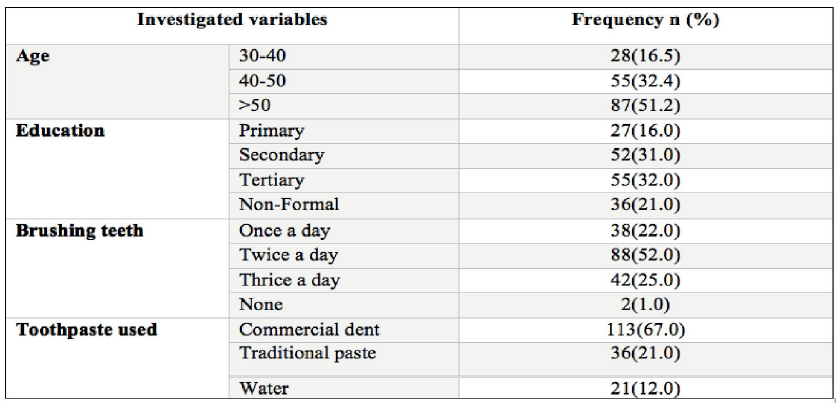
Table 1: Social demographic of the study participants
Periodontal problems in the study population
The loss of 1-3 teeth in patients with periodontal problems was mostly reported by 102 (60%). Halitosis was also observed in 133(66%) patients where teeth decay and gingivitis were more observed in patients with periodontal problems 98(58%) and 97(57%) respectively. Most patients reported to have no oral infection screening 143 (84%) (Table 2).
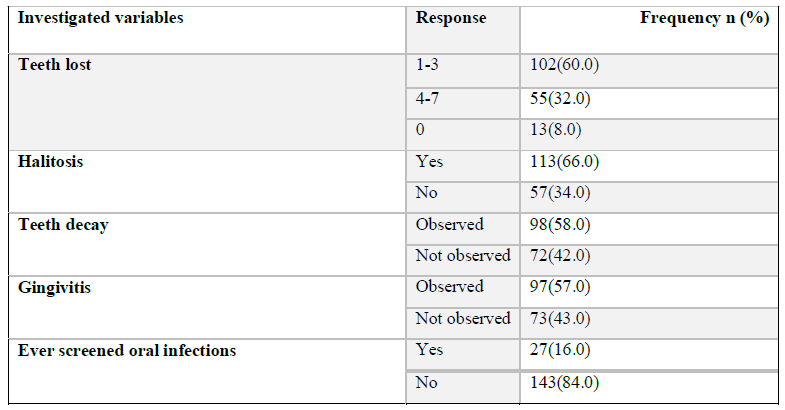
Table 2: Variables associated with periodontal problems in the study population
Isolation and identification of bacteria isolates
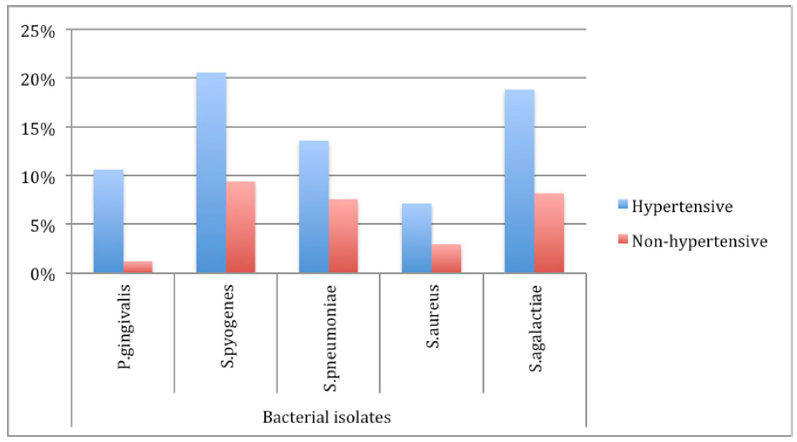
Of 170 randomly recruited and examined patients, were categorized into two groups namely HTP 120 (70.6%) and 50(29.4%) control group/NHTP respectively. A total of 111(65.3%) developed some kind of gum bleeding during supra and sub-gingival samples collection. From 170 patients, 5 species of bacteria were isolated from their mouths/sub-gingival swabs, which mostly were comprised of 20 (11.8%) P. gingivalis and 51(30.0%) S. pyogenes. Most of the isolated bacteria 120 (70.6%) were from the hypertensive patients; and of those 18(15.0%) were P. gingivalis isolates (Figure 1).
Figure 1: Bacteria isolated from patients’ sub-gingival swabs. Hypertensive patients recorded dominant carriage of transient oral bacterial flora, where S. pyogenes 20.6% (120) were mostly isolated and S. aureus 7.1% (120) were least isolated, while S. pyogenes 9.4% (50) was mostly isolated in non- hypertensive patients and P. gingivalis 1.2% (50) was least isolated.
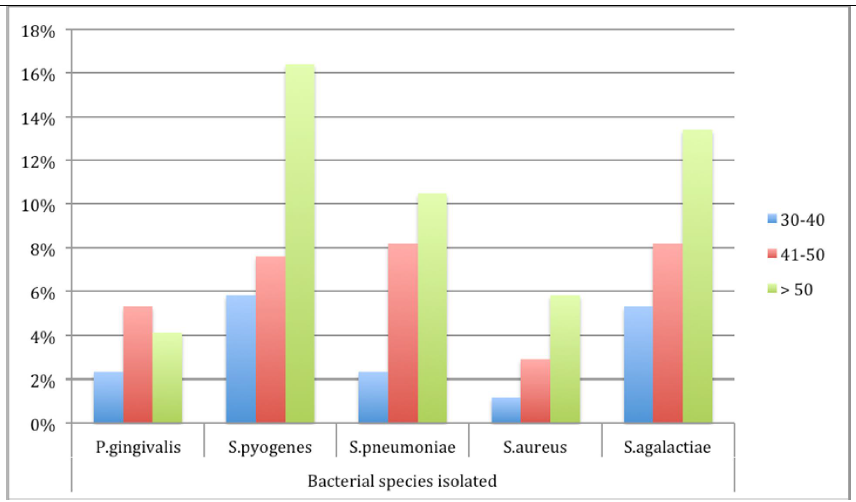
Bacteria isolated grouped per patients’ ages
A total 109 (63.7%) bacteria were isolated from female patients as compared to 61 (36.3%) from males. Of 18 isolates of P. gingivalis recovered from hypertensive patients’ sub-gingival swabs, 14 (77.8%) were from female patients. More bacteria were isolated from older patients over 50 years as compared to younger patients (17% vs. 50.3%; p=0.016) (Figure 2).
Regression analysis of social demographic factors
No direct association between gingivitis and HP was observed [Odds Ratio (OR) = 4.2; 95% Confidence Interval (95%CI) = 0.944-18.993; p = 0.130]. Interdependence of disease, patient’s sex and oral infection was revealed (Cramer’s V = 0.019, p = 0.02). Controlling for age, the OR to acquire HP was 0.084 for patients without formal education as compared to higher education earners [p = 0.024; 95%CI = 0.09 - 0.72]. On the other hand, age had no significant effect on the presence of oral P. gingivalis among the patients (p = 0.056). Neither teeth brushing habit or brushing frequency showed significant difference in relation to HP nor types of tooth paste used had significant effect on presence of oral bacteria; though patients who did not use toothpastes were more likely to harbor P. gingivalis (OR = 1.033; p > 0.05).
Discussions
Hypertension is a major risk factor affecting adult population over about 30 years of age. In Tanzania, hypertension ranks as the 17th disease that kills many people as consequence of direct disease condition of its associated complications such as stroke, myocardial infarction [1].
Researchers have shown that infections of the oral cavity such as gingivitis, periodontitis, or dental carries increase of systemic diseases like HP, as microorganisms spread throughout the body [18-20]. Most likely such diseases can indirectly involve systemic inflammation leading to release various inflammatory mediators that are part of microbial cellular components, chiefly lipo-polysaccharide that activate macrophages to synthesize several pro-inflammatory molecules, including the cytokines, prostaglandins, especially prostaglandin E2 and hydrolytic enzymes [21,22].
Since the oral cavity is an open system exposed to the external environment; possibilities of foreign materials entering the system from the oral cavity increase due to the constant food and liquids ingestion through the mouth. The presence of the large numbers of microorganisms, both pathogenic and normal flora can initiate tissue destruction by activating components of the immune system, which in turn, release mediators that evoke mechanisms of connective tissue degradation. Such microbial infections concomitantly affect the endothelium, monocytes and coagulation system/platelets that might be pro-atherogenic attributing indirectly to atherogenesis reducing intima wall thickness of spelling to rise of blood pressure.
Hence periodontal diseases can be result of chronic presence of P. gingivalis that emerge from the periodontal flora in susceptible individuals and can lead to frequent low-level bacteremia [23]. Consequently, recurrent bacteremia with P. gingivalis and a mixture of other oral bacteria can occur in periodontitis patients during simple mastication
- In this way, potential aassociations between gingivalis and HP that is backed up by our present findings and other epidemiological and experimental animal studies [25, 26]. We presume that P. gingivalis infections could be a factor that causes HP or health?related condition as its prevalence was direct proportional with frequency of HP. In order to establish a direct causality, stringent criteria must be met and such conditions need to occur before the disease outcome, which seems to be the case.
About half (57%) of the study population had gingivitis. Oral conditions such as gingivitis and chronic periodontitis are found worldwide and are among the most prevalent microbial diseases of mankind [27]. Other bacteria in the oral cavity have also proved a great deal attributing to HP including S. pyogenes and S. aureus, which have been reported to cause endocarditis and atherosclerosis [10,28]. The fact that periodontal disease is inflammatory in nature affecting up to 50% of the grown-ups [28]; thus inflammation is believed to exert a central role in the pathogenesis of atherosclerosis and other inflammatory diseases [27]. Nonetheless, this study couldn’t establish a direct association between gingivitis and HP, which explains that possible causes of gingivitis might be multi-factorial.
The isolation rate of P. gingivalis was relatively lower as compared to what was previously reported in Iran (11% vs. 37%) by [17]. However, our findings coincide with one previous study, which showed that health individuals had less possibility of being infected with P. gingivalis as compared to hypertensive ones [29]. A correlation between patients’ ages and frequency of bacteria isolation (51%) was apparent, which was more obvious among hypertensive patients over 50 years as compared to 24% previously observed [17]. Isolation method and human error could have played major role in the observed discrepancy. Prevalence of bacteria was higher among female patients than males coinciding with previous studies [10,17]. Among several factors, include presence of concomitant diseases among moist female patients; menopause and pregnancy that is accompanied with erosion of minerals from bones and teeth have been attributed to the higher prevalence of oral bacteria. Periodontal diseases were more prevalent in men than in women even though female patients were relatively more likely to suffer from HP. This relationship depends on socio-economic status, physiological processes such as bone metabolism, and the use of contraceptives [30,31]. Associations between level of education and HP as well as between halitosis and HP were evident, which coincided with a study by Milanesi et al., (2016) [32]. The fact that our study found that younger people were more likely to suffer from HP as compared to older ones could be attributed to lifestyle and stress among the youth. Similarly, lifestyle and levels of education seem to play important role in oral health; as only half of the participants brushed their teeth twice a day and of these, 63% had at least secondary education.
LimitationsOccasionally, collection of required sub-gingival fluid or plaque specimens was not possible, only saliva specimens were obtained thus deterring bacteria identification as either pathogenic or normal flora. Patients’ disinclination to disclose some life-styles and healthy-related information that could have assisted to better describe the association of bacterial infection and HP. Concurrent determination of HP and oral infection causing bacteria, might have affected the progression and thus inability to establish underlying factors. Nonetheless, correction for confounders through statistical adjustment has minimized effects of other factors.
Conclusion
Hypertensive patients exhibit high oral carriage of transient bacteria especially P. gingivalis as compared to non-hypertensive individuals. However, there was no association between P. gingivalis carriage and hypertension.. Oral infections and other periodontal diseases were associated with HP. However, no conclusive remarks can be made regarding the causative involvement of periodontal diseases mainly due to the reduced number of available prospective studies. Oral health education should be disseminated among hypertensive patients and general public in line with regular dental examinations to avoid potential emergence of cardiovascular problems including hypertension as consequence of otherwise preventable oral bacterial infections.
Acknowledgements
We are grateful to patients who consented to participate in this study and thanks to all Clinicians and Laboratory personnel at MNH for their different forms of support during the phase of data collection.
References
- Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 21 (2017): e20171904.
- Kharlamova N, Jiang X, Sherina N, Potempa B, Israelsson L, et al. Antibodies to Porphyromonas gingivalis indicate interaction between oral infection, smoking, and risk genes in rheumatoid arthritis etiology. Arthritis & rheumatology 68 (2016): 604-613.
- Malha L, August P. Secondary hypertension in pregnancy. Current hypertension reports 17 (2015): 53.
- Grossman A, Messerli FH, Grossman E. Drug induced hypertension–An unappreciated cause of secondary hypertension. European journal of pharmacology 763 (2015): 15-22.
- Kishore J, Gupta N, Kohli C, Kumar N. Prevalence of hypertension and determination of its risk factors in rural Delhi. International journal of hypertension. 2016: 2016.
- Rahimi K, Emdin CA, MacMahon S. The epidemiology of blood pressure and its worldwide management. Circulation research 116 (2015): 925-936.
- Gerits, E.; Verstraeten, N.; Michiels, J. New approaches to combat porphyromonas gingivalis biofilms. J Oral Microbiol 9 (2017): 1300366.
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat Rev Immunol 15 (2015): 30–44.
- Zhang B, Khalaf H, Sirsjö A, Bengtsson T. Gingipains from the periodontal pathogen Porphyromonas gingivalis play a significant role in regulation of angiopoietin 1 and angiopoietin 2 in human aortic smooth muscle cells. Infection and Immunity 17 (2017): IAI-00498.
- Desvarieux M, Demmer RT, Jacobs Jr DR, Rundek T, Boden-Albala B, Sacco RL, Papapanou PN. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). Journal of hypertension 28 (2010): 1413.
- Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nature Reviews Rheumatology 13 (2017): 606.
- Cheesbrough M. District laboratory practice in tropical countries. Cambridge university press 2006.
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision. Available at:Worldometers (Worldometers.info)
- Fleming SM, O'Byrne L, Grimes H, Daly KM, Morrison JJ, Morrison JJ. Amino-terminal pro-brain natriuretic peptide in normal and hypertensive pregnancy. Hypertension in pregnancy 20 (2001): 169-175.
- To TT, Gümü? P, Nizam N, Buduneli N, Darveau RP. Subgingival plaque in periodontal health antagonizes at toll-like receptor 4 and inhibits e-selectin expression on endothelial cells. Infection and immunity 84 (2016): 120-126.
- Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. ArteriosclerThromb Vasc Biol 23 (2003): 1245–1249
- Japoni A, Vazin A, Noushadi S, Kiany F, Japoni S, Alborzi A. Antibacterial susceptibility patterns of Porphyromonas gingivalis isolated from chronic periodontitis patients. Med Oral Patol Oral Cir Bucal 16 (2011): 1031–1035.
- Willems HM, Xu Z, Peters BM. Polymicrobial biofilm studies: from basic science to biofilm control.Current oral health reports 3 (2016): 36-44.
- Shiheido Y, Maejima Y, Suzuki JI, Aoyama N, Kaneko M, et al. Porphyromonas gingivalis, a periodontal pathogen, enhances myocardial vulnerability, thereby promoting post-infarct cardiac rupture. Journal of molecular and cellular cardiology 99 (2016): 123-137.
- Holmlund A, Hedin M, Pussinen PJ, Lerner UH, Lind L. Porphyromonas gingivalis (Pg) a possible link between impaired oral health and acute myocardial infarction. International journal of cardiology 148 (2011): 148-153.
- Chistiakov DA, Orekhov AN, Bobryshev YV. Links between atherosclerotic and periodontal disease. Experimental and Molecular Pathology 100 (2016): 220-235.
- Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clinical microbiology reviews 13 (2000): 547-558.
- Loesche WJ. Periodontal disease: link to cardiovascular disease. Compend Contin Educ Dent 21 (2000): 463–466, 468, 470 passim; quiz 484.
- Maharaj B, Coovadia Y, Vayej AC. An investigation of the frequency of bacteraemia following dental extraction, tooth brushing and chewing. Cardiovascular Journal of Africa 23 (2012): 340.
- Supuran CT, Capasso C. Carbonic anhydrase from Porphyromonas gingivalis as a drug target. Pathogens 6 (2017): 30.
- Pussinen PJ, Alfthan G, Tuomilehto J, Asikainen S, Jousilahti P. High serum antibody levels to Porphyromonas gingivalis predict myocardial infarction. European Journal of Cardiovascular Prevention & Rehabilitation 11 (2004): 408-411.
- Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clinical Microbiology and Infection 13 (2007): 3-10.
- Belstrøm D, Damgaard C, Nielsen CH, Holmstrup P. Does a causal relation between cardiovascular disease and periodontitis exist? Microbes and infection 14 (2012): 411-418.
- Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontology 62 (2013): 59-94.
- Leng B, Jin Y, Li G, Chen L, Jin N. Socioeconomic status and hypertension: a meta-analysis. Journal of hypertension 33 (2015): 221-229.
- Meisel P, Reifenberger J, Haase R, Nauck M, Bandt C, Kocher T. Women are periodontally healthier than men, but why don't they have more teeth than men? Menopause 15 (2008): 270-275.
- Milanesi FC, Kauer B, Wagner TP, Daudt LD, Haas AN. Self-reported halitosis and associated demographic and behavioral factors. Brazilian oral research 30 (2016).