Association of Serum Vascular Endothelial Growth Factor (VEGF) with Colorectal Cancer: A Systemic Review and Meta-Analysis
Article Information
Donglie Zhu1#, Zelong Yang1#, Xiangxia Miao2#, Wanli Yang3#, Mingzuo Jiang3, Yong Chen1*
#These authors contributed equally to this work
1Department of Hepatobiliary Surgery, Fourth Military Medical University, Shaanxi, China
2Department of Respiratory Medicine, The First Hospital of Yulin, Shaanxi, China
3State key Laboratory of Cancer Biology, Fourth Military Medical University, Shaanxi, China
*Corresponding Author: Yong Chen, Department of Hepatobiliary Surgery, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi, 710032, China
Received: 15 November 2019; Accepted: 27 November 2019 Published: 02 January 2020;
Citation: Donglie Zhu, Zelong Yang, Xiangxia Miao,Wanli Yang, Mingzuo Jiang, Yong Chen. Association of Serum Vascular Endothelial Growth Factor (VEGF) with Colorectal Cancer: A Systemic Review and Meta-Analysis. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 015-031.
View / Download Pdf Share at FacebookAbstract
Vascular endothelial growth factor (VEGF) is one of the most important regulators in angiogenesis, affecting endothelial cell survival and function. Some studies have shown that serum VEGF is higher in CRC patients than in healthy control groups while other studies have given the opposite conclusion. Therefore, this meta-analysis is purposed to systemically review and evaluate the correlation between serum VEGF and CRC. Finally, 23 studies were included in this study. The meta-analysis demonstrated that serum VEGF in the cancer group was significantly higher than that in the control group (SMD: 1.5, 95% CI: 1.05-1.95, P<0.001). However, obvious heterogeneity existed among the studies (P<0.001, I2=96%) and subgroup analyses were performed to investigate the source of this heterogeneity. The results indicated that with respect to VEGF, the correlation was significant regarding tumor location, study region, age, and study size. The results of this meta-analysis showed that serum level of VEGF might be used as a candidate biomarker for CRC patients.
Keywords
VEGF; CRC; Meta-analysis; Heterogeneity
VEGF; CRC articles, Meta-analysis articles, Heterogeneity articles
VEGF articles VEGF Research articles VEGF review articles VEGF PubMed articles VEGF PubMed Central articles VEGF 2023 articles VEGF 2024 articles VEGF Scopus articles VEGF impact factor journals VEGF Scopus journals VEGF PubMed journals VEGF medical journals VEGF free journals VEGF best journals VEGF top journals VEGF free medical journals VEGF famous journals VEGF Google Scholar indexed journals CRC articles CRC Research articles CRC review articles CRC PubMed articles CRC PubMed Central articles CRC 2023 articles CRC 2024 articles CRC Scopus articles CRC impact factor journals CRC Scopus journals CRC PubMed journals CRC medical journals CRC free journals CRC best journals CRC top journals CRC free medical journals CRC famous journals CRC Google Scholar indexed journals Meta-analysis articles Meta-analysis Research articles Meta-analysis review articles Meta-analysis PubMed articles Meta-analysis PubMed Central articles Meta-analysis 2023 articles Meta-analysis 2024 articles Meta-analysis Scopus articles Meta-analysis impact factor journals Meta-analysis Scopus journals Meta-analysis PubMed journals Meta-analysis medical journals Meta-analysis free journals Meta-analysis best journals Meta-analysis top journals Meta-analysis free medical journals Meta-analysis famous journals Meta-analysis Google Scholar indexed journals Heterogeneity articles Heterogeneity Research articles Heterogeneity review articles Heterogeneity PubMed articles Heterogeneity PubMed Central articles Heterogeneity 2023 articles Heterogeneity 2024 articles Heterogeneity Scopus articles Heterogeneity impact factor journals Heterogeneity Scopus journals Heterogeneity PubMed journals Heterogeneity medical journals Heterogeneity free journals Heterogeneity best journals Heterogeneity top journals Heterogeneity free medical journals Heterogeneity famous journals Heterogeneity Google Scholar indexed journals Colorectal cancer articles Colorectal cancer Research articles Colorectal cancer review articles Colorectal cancer PubMed articles Colorectal cancer PubMed Central articles Colorectal cancer 2023 articles Colorectal cancer 2024 articles Colorectal cancer Scopus articles Colorectal cancer impact factor journals Colorectal cancer Scopus journals Colorectal cancer PubMed journals Colorectal cancer medical journals Colorectal cancer free journals Colorectal cancer best journals Colorectal cancer top journals Colorectal cancer free medical journals Colorectal cancer famous journals Colorectal cancer Google Scholar indexed journals Angiogenesis articles Angiogenesis Research articles Angiogenesis review articles Angiogenesis PubMed articles Angiogenesis PubMed Central articles Angiogenesis 2023 articles Angiogenesis 2024 articles Angiogenesis Scopus articles Angiogenesis impact factor journals Angiogenesis Scopus journals Angiogenesis PubMed journals Angiogenesis medical journals Angiogenesis free journals Angiogenesis best journals Angiogenesis top journals Angiogenesis free medical journals Angiogenesis famous journals Angiogenesis Google Scholar indexed journals colonoscopy articles colonoscopy Research articles colonoscopy review articles colonoscopy PubMed articles colonoscopy PubMed Central articles colonoscopy 2023 articles colonoscopy 2024 articles colonoscopy Scopus articles colonoscopy impact factor journals colonoscopy Scopus journals colonoscopy PubMed journals colonoscopy medical journals colonoscopy free journals colonoscopy best journals colonoscopy top journals colonoscopy free medical journals colonoscopy famous journals colonoscopy Google Scholar indexed journals fecal immunochemical tests articles fecal immunochemical tests Research articles fecal immunochemical tests review articles fecal immunochemical tests PubMed articles fecal immunochemical tests PubMed Central articles fecal immunochemical tests 2023 articles fecal immunochemical tests 2024 articles fecal immunochemical tests Scopus articles fecal immunochemical tests impact factor journals fecal immunochemical tests Scopus journals fecal immunochemical tests PubMed journals fecal immunochemical tests medical journals fecal immunochemical tests free journals fecal immunochemical tests best journals fecal immunochemical tests top journals fecal immunochemical tests free medical journals fecal immunochemical tests famous journals fecal immunochemical tests Google Scholar indexed journals biomarker articles biomarker Research articles biomarker review articles biomarker PubMed articles biomarker PubMed Central articles biomarker 2023 articles biomarker 2024 articles biomarker Scopus articles biomarker impact factor journals biomarker Scopus journals biomarker PubMed journals biomarker medical journals biomarker free journals biomarker best journals biomarker top journals biomarker free medical journals biomarker famous journals biomarker Google Scholar indexed journals serum level articles serum level Research articles serum level review articles serum level PubMed articles serum level PubMed Central articles serum level 2023 articles serum level 2024 articles serum level Scopus articles serum level impact factor journals serum level Scopus journals serum level PubMed journals serum level medical journals serum level free journals serum level best journals serum level top journals serum level free medical journals serum level famous journals serum level Google Scholar indexed journals
Article Details
1. Introduction
Colorectal cancer (CRC) is one of the most frequently diagnosed cancers [1, 2]. It has been reported that about 1 million people develop CRC each year worldwide (3). Moreover, the disease-related mortality of CRC patients corresponds to about 33% [3]. Several factors have been found to be associated with CRC, including inflammatory bowel disease, CRC history in first-degree relatives, obesity, little physical activity, smoking and high intake of red meat [4]. And screening is an effective way to reduce the related mortality of CRC through colonoscopy and fecal immunochemical tests (FIT) [5]. Angiogenesis plays a critical role in tumor development and angiogenic factors are important targets of tumor therapy. As we know, a plenty of cytokines are involved in tumor angiogenesis, such as VEGF, Ang1, PDGF-A, PDGF-B, and IL-8 [6, 7]. VEGF has a close relationship with multiple kinds of tumors and it has been reported that VEGF has been described as overexpressed in lung cancer [8]. In addition, anti-VEGF treatment is proven to relieve nerve edema and deliver oxygen more efficiently into nerves to improve the nerve function of patients who have had / (or have) tumors of the nervous system [9]. In the digestive system, a high level of VEGF is associated with the development of CRC [10]. VEGF is a multi-functional cytokine and mainly acts on the vascular endothelium. According to the studies, VEGF can induce the mitotic activity in endothelial cells and capillary sprouting transferred by two high-affinity receptors (Flt-1 and Flk-1/KDR) [11, 12]. It has been reported that serum VEGF has a strong relationship with the CRC. However, whether CRC patients have a high level of serum VEGF or not remains to be determined. For example, Bünger S, et al. found that serum VEGF was obviously lower in CRC patients compared with healthy control groups [13]. Nevertheless, Landriscina, et al. demonstrated that serum VEGF is not significantly correlated with CRC [14]. Therefore, we conducted this analysis so as to assess the correlations between the serum VEGF and CRC. We also aim to clarify the role of serum VEGF in CRC development, and finally provide evidence for further studies and novel therapeutic methods for CRC patients through targeting VEGF.
2. Methods
2.1 Literature search
A comprehensive literature search was carried out using online databases including PubMed, EMBASE, and Web of Science. Key words for the literature search were as follows: “serum”, “serum level”, “vascular endothelial growth factor”, “VEGF”, “colorectal”, “CRC”, “colon”, “cancer” and “tumor”. The above search terms were only for human subjects. We searched for full-text articles and abstracts published in English and all relevant articles that were identified online were from March 1998 to June 2018. All potentially applicable studies were considered for review, regardless of the primary outcome. The full-text articles were screened independently by 2 members of the research team, and another member of the research team resolve any disagreements; the third member also reviewed all the excluded articles. We also performed a manual search in reference lists in order to find the additional relevant papers.
2.2 Inclusion and exclusion criteria
Three authors independently screened the suitable study records. Studies that met the following criteria could be included: (1) the cases of patients diagnosed with CRC where the controls were healthy people (2) reported serum VEGF levels in CRC patients and healthy controls; (3) studies that provided the means (M) and standard deviation (SD) of the serum VEGF levels in CRC patients and controls; (4) studies published in English. The exclusion criteria were based on the following: (1) the articles were reviewed, case report, abstract, or unpublished papers; (2) patients’ sample were not serum source; (3) studies without complete data.
2.3 Data extraction
Three researchers independently elicited the data of the included articles: the study title, name of first authors, year of publication, country, type of study, age and sex of participants, sample size, mean ± SD of VEGF levels, and clinical characteristics of participants.
2.4 Quality assessment
We adopted the Newcastle Ottawa Scale (NOS) to conduct the quality assessment independently by two researchers. The NOS tool contains nine items and each study was evaluated by an NOS score ranging from 0 to 9. We defined studies as poor quality if the NOS score ≤ 3, 4 to 6 corresponds to moderate quality and studies were high quality when their score was from 7 to 9.
2.5 Statistical analysis
Subgroup analysis and meta regression have been used for heterogeneity analysis. The heterogeneity of the studies was evaluated by the SMD and 95% CI. Significant heterogeneity was found in the studies by a p value < 0.1 for Q test or I2 > 50%. Heterogeneity among the studies was tested by using a random effect model when indicated. Publication bias was investigated both visually by using a funnel plot and statistically via Begg funnel plots and the Eggers bias test, which measures the degree of funnel plot asymmetry. RevMan 5.3 and State SE11.0 were performed for all analyses.
3. Results
3.1 Literature search
According to the search criteria, in total, 593 studies were collected by the literature search. 268 relevant articles after duplicates removed. 169 articles were excluded, 99 articles assessed for eligibility. Among them, 76 articles were excluded according to the exclusion criteria (25 papers were reviewed, 27 papers had no extractable date, 16 papers were without control groups, and 8 papers had sample overlap). Finally, 23 studies with 3400 subjects (2510 CRC patients and 890 controls) were included in this meta-analysis. Figure 1 presents the process of student selection.
3.2 Characteristics of the included studies
As shown in Table 1, we summarized the main characteristics of the included studies. The studies were published between March 1998 to June 2018 and contain 2510 CRC cases and 890 healthy people. All studies used serum samples. The enzyme-linked immune Sorbent assay (ELISA) was used to detect the VEGF levels. Thirteen studies were performed in Europe, nine studies were performed in Asia, and one was in Egypt. The 23 studies contain twenty-one case-control studies and two prospective studies. In accordance to NOS: five studies scored 5 [15-19], eleven studies scored 6 [13, 14, 20-28] and seven studies scored 7 [29-35] (Table 1).
3.3 Meta-analysis
As shown in Figure 2, The overall effect indicated that VEGF levels in CRC patients were strongly higher than that in healthy cases (SMD: 1.5, 95% CI: 1.05-1.95, P<0.001) according to the results, the heterogeneity across studies was significant (P<0.001, I2=96%), therefore we performed the random effect model.
3.4 Subgroup analysis
Subgroup analysis was carried out to investigate the heterogeneity among studies and evaluate the robustness of our findings. Tumor location, region and sample size of the studies were used to evaluate potential sources of heterogeneity (Table 2). Following tumor locations, all studies were divided into either a colon group or others, eight studies were reported in the colon group and seventeen studies were reported in others. As shown in Figure 3, higher VEGF levels were detected in case compared with the control both in colon (SMD: 2.93, 95% CI: 1.65–4.20, P<0.0001) and others subgroup (SMD: 1.17, 95% CI:0.71–1.63, P<0.0001). Subgroup analysis which was based on the region of studies revealed that serum VEGF in CRC patients was higher than healthy control groups Asia: SMD: 1.36, 95% CI: 0.62-2.09, P=0.0003, Europe: SMD: 1.61, 95% CI: 1.02-2.20, P<0.0001) (Figure 4). Further subgroup analysis which was based on the sample size revealed that serum VEGF in CRC patients was higher than healthy controls in both the two subgroups (sample size < 50: SMD: 3.23, 95% CI: 1.81-4.64, P<0.0001; sample size ≥ 50: SMD: 0.91, 95% CI: 0.50-1.33, P<0.0001) (Figure 5). Moreover, studies which were based on age showed that serum VEGF in CRC patients was higher than the healthy controls (age < 50: SMD: 2.21, 95% CI: 1.07-3.34, P=0.0001, age ≥ 50: SMD: 0.96, 95% CI: 0.41-1.52, P=0.0007) (Figure 6). Still, all subgroup analysis showed large heterogeneity, and these variables did not contribute to finding the source of heterogeneity.
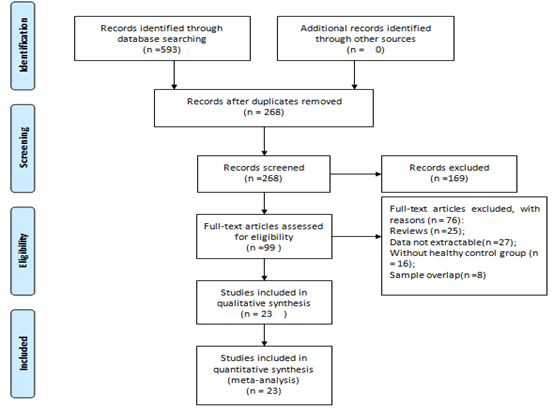
Figure 1: Flow diagram of literature search and selection.
Abbreviations: NOS=Newcastle-Ottawa quality assessment scale; ELISA=enzyme-linked immune Sorbent assay; VEGF= Vascular endothelial growth factor.
Table 1: Characteristics of the included studies.
Abbreviations: SMD=Standard Mean Difference; CI= Confidence Intervals.
Table 2: Subgroup analysis of VEGF level in CRC.

Figure 2: SMD analysis of serum VEGF level in CRC patients and the controls.
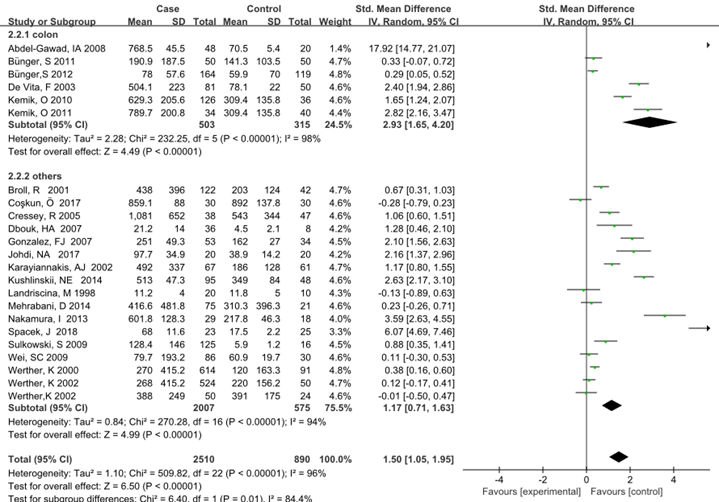
Figure 3: Subgroup analyses for relationship between serum VEGF and CRC according to tumor location.
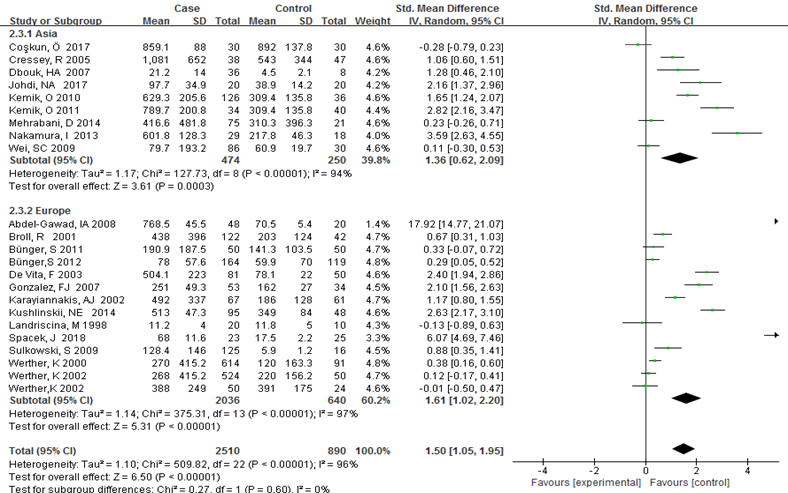
Figure 4: Subgroup analyses for relationship between serum VEGF and CRC according to region.
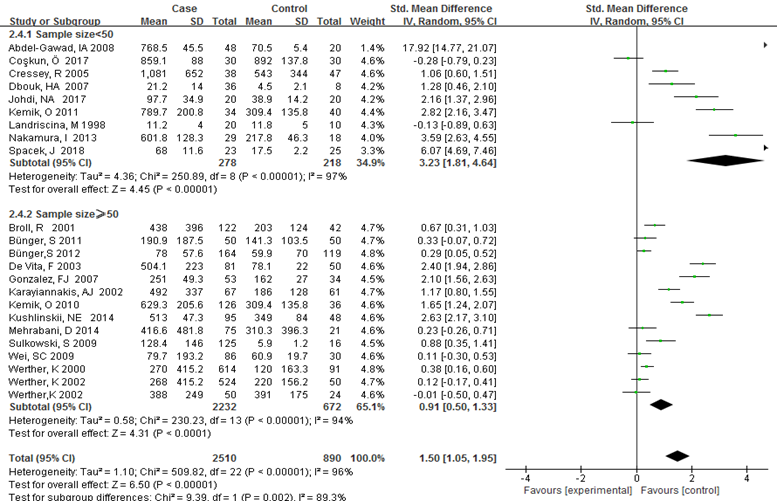
Figure 5: Subgroup analyses for relationship between serum VEGF and CRC according to sample size
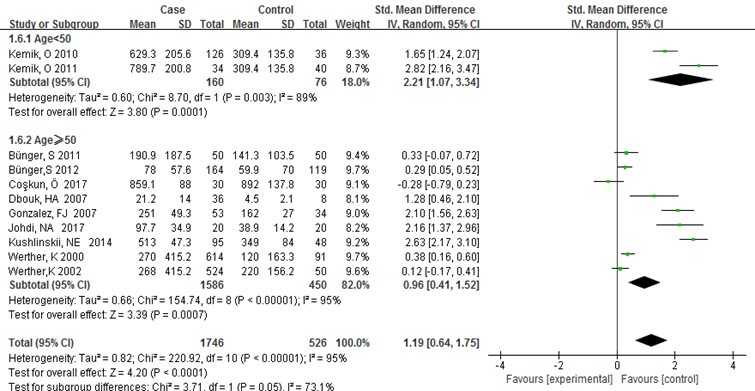
Figure 6:Subgroup analyses for relationship between serum VEGF and CRC according to age.
3.5 Sensitivity
Sensitivity analysis was performed to examine the resulting stability, in which one study was removed at a time. The results suggested that the omission of any study had no obvious effect on the findings, which also reflected the robustness of the conclusions (Table 3).
|
Study |
SMD (95% CI) |
P heterogeneity |
I2 |
|
Abdel-Gawad et al. |
1.24 (0.84-1.64) |
P<0.0001 |
95% |
|
Broll et al. |
1.56(1.08-2.04) |
P<0.0001 |
96% |
|
Bünger et al. |
1.57(1.09-2.04) |
P<0.0001 |
96% |
|
Bünger et al. |
1.59(1.10-2.07) |
P<0.0001 |
96% |
|
Co?kun et al. |
1.59(1.12-2.05) |
P<0.0001 |
96% |
|
Cressey et al. |
1.53(1.06-2.00) |
P<0.0001 |
96% |
|
Dbouk et al. |
1.51(1.05-1.98) |
P<0.0001 |
96% |
|
De Vita et al. |
1.44(0.99-1.89) |
P<0.0001 |
95% |
|
Gonzalez et al. |
1.47(1.01-1.93) |
P<0.0001 |
96% |
|
Johdi et al. |
1.47(1.01-1.93) |
P<0.0001 |
96% |
|
Karayiannakis et al. |
1.53(1.05-2.00) |
P<0.0001 |
96% |
|
Kemik et al. |
1.50(1.03-1.96) |
P<0.0001 |
96% |
|
Kemik et al. |
1.43(0.98-1.88) |
P<0.0001 |
96% |
|
Kushlinskii et al. |
1.43(0.98-1.87) |
P<0.0001 |
95% |
|
Landriscina et al. |
1.57(1.11-2.04) |
P<0.0001 |
96% |
|
Mehrabani et al. |
1.57(1.10-2.04) |
P<0.0001 |
96% |
|
Nakamura et al. |
1.40(0.95-1.85) |
P<0.0001 |
96% |
|
Spacek et al. |
1.32(0.89-1.76) |
P<0.0001 |
95% |
|
Sulkowski et al. |
1.54(1.07-2.01) |
P<0.0001 |
96% |
|
Wei et al. |
1.57(1.10-2.04) |
P<0.0001 |
96% |
|
Werther et al. |
1.59(1.09-2.08) |
P<0.0001 |
96% |
|
Werther et al. |
1.58(1.11-2.04) |
P<0.0001 |
96% |
|
Werther et al. |
1.58(1.11-2.06) |
P<0.0001 |
96% |
Abbreviations: SMD= Standard Mean Difference; CI= Confidence Intervals.
Table 3: Sensitivity analysis.
3.6 Meta regression
To determine the source of heterogeneity, meta regression analyses were performed. The results of random-effects model meta-regression based on age, published year, region, design, NOS, and the sample size showed that they cannot explain the heterogeneity of the included studies. Table 4 presented the results of univariate analysis. Only
region was considered as a key factor that might be weakly responsible for the heterogeneity among the included studies. After introducing all the covariates, the heterogeneity changes from 96% to 96.19% and the covariates did not contribute to heterogeneity in any of the preplanned comparisons (Table 4).
|
Covariates |
No. of studies |
Coefficient |
Standard error |
t |
P |
95% Confidence interval |
|
|
Age |
11 |
-1.251 |
0.824 |
-1.52 |
0.163 |
-3.116 |
0.613 |
|
Year |
23 |
0.110 |
0. 128 |
0.86 |
0.397 |
-0.156 |
0. 378 |
|
Region |
23 |
2.368 |
1.230 |
1.92 |
0.068 |
-0.191 |
4.927 |
|
Design |
23 |
0.017 |
2.663 |
0.01 |
0.995 |
-5.520 |
5.554 |
|
NOS |
23 |
1.614 |
0.980 |
1.65 |
0.115 |
-0.425 |
3.653 |
|
Sample size |
23 |
-0.254 |
1.821 |
-0.14 |
0.890 |
-4.042 |
3.532 |
Table 4: Univariate meta-regression analysis for the potential variables between studies.
3.7 Publication bias
We conducted the funnel plot analysis and Egger’s linear regression test to explore the potential publication bias. The Egger’s (t=-1.80, P=0.087) suggested that there were no significant publication biases in the included studies (Figure 7).
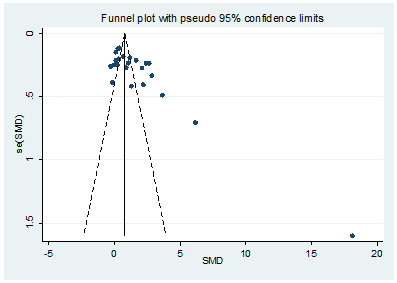
Figure 7: A funnel plot analysis of publication bias.
4. Discussion
CRC is one of the gastrointestinal cancers with the highest incidence [36]. Angiogenesis is an essential process for the growth and proliferation of cancer [37]. Numerous experimental and clinical studies have shown that high serum VEGF expression is related to CRC development. VEGF is a glycosylated dimeric polypeptide which is abundantly expressed and secreted by most human tumors. Some studies have found that VEGF can be a potential serum diagnostic marker for malignant disease since high VEGF mRNA levels can be detected in most human tumors by situ hybridization [38]. Furthermore, studies have also shown that VEGF and its receptors? are highly expressed in metastatic colon cancer cells and tumor associated endothelial cells [39]. These findings indicate that VEGF is an important angiogenic factor in CRC development [40]. Currently, many researchers are focusing on the association of serum VEGF and CRC. However, the results might be contradictory. For example, Spacek et al. have pointed out that serum VEGF and CRC are positively correlated [35]. While some studies gave different results. Co?kun et al. found that serum VEGF was lower in CRC patients compared with the healthy control groups [30].To the best of our knowledge, this is the first systematic analysis to explore the relationship of serum VEGF level and CRC. In this study, our results have shown that CRC patients had a higher serum VEGF level than the healthy controls. When interpreting these findings, we should take the following issues into consideration. First, VEGF plays an important role in CRC cells through the intracrine mechanism, that is, by regulating the activity of multiple receptor tyrosine kinases and downstream AKT signaling [10]. Second, inhibition of intracrine VEGF pathways can strongly suppress CRC invasion and migration via modulating the cell motility related molecules [41]. Third, Chronic inhibition of extracellular VEGF resulted in resistance to hypoxia-induced apoptosis and an increased sphere formation ability in CRC cell lines [42].
To explore the heterogeneity among studies, we performed subgroup analyses according to tumor location, region, sample size and age. It is known that VEGF polymorphism was found to be associated with malignancy susceptibility in CRC [43], and different types of gastrointestinal cancers that have disparate mechanisms of a carcinogenic effect. Therefore, we stratified subgroup analysis according to tumor location (Table 2). The results showed that the serum VEGF level was significantly higher in case groups than that in the control groups. However, the heterogeneity was obvious. In addition, Age is the main risk factor for CRC. It is reported that the risk of CRC increases significantly when people are over 50 years old [44]. Therefore, we performed subgroup analyses based on age, whether the age was <50 or ≥ 50. However, heterogeneity significantly still existed. Moreover, subgroup analysis, which was based on region and sample size revealed that serum VEGF in CRC patients was obviously higher than in the healthy controls and the heterogeneity still existed. According to the sensitivity analysis, the correlation was stable. In order to find out the source of heterogeneity, meta regression analysis was performed, the results showed that the region might be weakly responsible for the overall heterogeneity (P=0.068). Still, this study had several limitations. Firstly, significant heterogeneity existed among the studies. Although we have performed subgroup analysis, sensitivity analysis and regression analysis to explore the source of heterogeneity, the explanation was unsatisfactory. Secondly, our study was based on unadjusted estimates. And the confounding factors such as tumor stage, before and after intervention, and environmental factors should be considered. Thirdly, the sizes of subjects in several studies were small, and the backgrounds of patients varied, which might cause a lower statistical power and even the inconsistent results. Finally, we should be cautious in drawing conclusions with these limitations. Our study found that the serum level of VEGF in CRC patients was obviously high compared to healthy controls, suggesting that VEGF can be a potential serum diagnostic marker for CRC. In order to investigate whether high VEGF levels are affected by the region, larger sample sizes and adjustments to mixing factors need to be designed.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (Nos. 81670563).
Compliance with Ethics Guidelines
Not required.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136 (2015): E359-E386.
- Torre LA, Bray F, Siegel RL, et al. Global Cancer Statistics. CA CANCER J CLIN 65 (2012): 69-90.
- Testa U, Pelosi E, Castelli G. Colorectal Cancer: Genetic Abnormalities, Tumor Progression, Tumor Heterogeneity, Clonal Evolution and Tumor-Initiating Cells. Med Sci (Basel) 6 (2018): 31-144.
- Johnson CM, Wei C, Ensor JE, et al. Meta-analyses of Colorectal Cancer Risk Factors. Cancer Causes Control 24 (2013): 1207-1222.
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 112 (2017): 1016-1030.
- Angelucci A, Monache SD, Cortellini A, et al. “Vessels in the Storm”: Searching for Prognostic and Predictive Angiogenic Factors in Colorectal Cancer. Int J Mol Sci 19 (2018): 299.
- Kahlert C, Fiala M, Musso G, et al. Prognostic impact of a compartment-specific angiogenic marker profile in patients with pancreatic cancer. Oncotarget 5 (2014): 12978-12989.
- Frezzetti D, Gallo M, Maiello MR, et al. VEGF as a potential target in lung cancer. Expert Opin Ther Targets 21 (2017): 959-966.
- Zhang N, Chen J, Ferraro GB, et al. Anti-VEGF treatment improves neurological function in tumors of the nervous system. Exp Neurol 299 (2018): 326-333.
- Bhattacharya R, Ye XC, Wang R, et al. Intracrine VEGF Signaling Mediates the Activity of Prosurvival Pathways in Human Colorectal Cancer Cells. Cancer Res 76 (2016): 3014-3024.
- Psatha A, Makris D, Kerenidi T, et al. A potential role for VEGF in the diagnostic approach of pleural effusions. J Thorac Dis 8 (2016): 1681-1687.
- Røe OD, Stella GM. Malignant pleural mesothelioma: History, controversy and future of a manmade epidemic. Eur Respir Rev 24 (2015): 115-131.
- Bünger S, Haug U, Kelly FM, et al. Toward Standardized High-Throughput Serum Diagnostics: Multiplex–Protein Array Identifies IL-8 and VEGF as Serum Markers for Colon Cancer. J Biomol Screen 16 (2011): 1018-1026.
- Landriscina M, Cassano A, Ratto C, et al. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. Br J Cancer 78 (1988): 765-770.
- Broll R, Erdmann H, Duchrow M, et al. Vascular endothelial growth factor (VEGF) -a valuable serum tumour marker in patients with colorectal cancer?. Eur J Surg Oncol 27 (2001): 37-42.
- Cressey R, Wattananupong O, Lertprasertsuke N, et al. Alteration of protein expression pattern of vascular endothelial growth factor (VEGF) from soluble to cell-associated isoform during tumourigenesis. BMC Cancer 5 (2005): 128-136.
- Nakamura I, Shibata M, Gonda K, et al. Serum levels of vascular endothelial growth factor are increased and correlate with malnutrition, immunosuppression involving MDSCs and systemic inflammation in patients with cancer of the digestive system. Oncol Lett 5 (2013): 1682-1686.
- Wei SC, Liang JT, Tsao PN, et al. Preoperative Serum Placenta Growth Factor Level Is a Prognostic Biomarker in Colorectal Cancer. Dis Colon Rectum 9 (2009): 1630-1636.
- Werther K, Christensen IJ, Nielsen HJ. Determination of vascular endothelial growth factor (VEGF) in circulating blood: significance of VEGF in various leucocytes and platelets. Scand J Clin Lab Invest 62 (2002): 343-350.
- Bünger S, Haug U, Kelly M, et al. A novel multiplex-protein array for serum diagnostics of colon cancer: a case–control study. BMC Cancer12 (2012): 393-405.
- Gonzalez FJ, Quesada AR, Sevilla I, et al. Prognostic value of serum angiogenic activity in colorectal cancer patients. J Cell Mol Med 1 (2007): 120-128.
- Johdi NA, Mazlan L, Mazlan L, et al. Profiling of cytokines, chemokines and other soluble proteins as a potential biomarker in colorectal cancer and polyps. Cytokine 99 (2017): 35-42.
- Kemik O, Sumer A, Kemik AS, et al. The relationship among acute-phase response proteins, cytokines and hormones in cachectic patients with colon cancer. World J Surg Oncol 8 (2010): 85-91.
- Kemik O, Kemik AS, Begenik H, et al. The relationship among acute-phase responce proteins, cytokines, and hormones in various gastrointestinal cancer types patients with cachectic. Hum Exp Toxicol 2 (2012): 117-125.
- Mehrabani D, Shamsdin SA, Dehghan A, et al. Clinical Significance of Serum Vascular Endothelial Growth Factor and Complement 3a Levels in Patients with Colorectal Cancer in Southern Iran. Asian Pac J Cancer Prev 22 (2014): 9713-9717.
- Sulkowski S, Wincewicz A, Zalewski B, et al. Hypoxia related growth factors and p53 in preoperative sera from patients with colorectal cancer-evaluation of the prognostic significance of these agents. Clin Chem Lab Med 11 (2009):1439-1445.
- Werther K, Christensen IJ, Brünner N, et al. Soluble vascular endothelial growth factor levels in patients with primary colorectal carcinoma. Eur J Surg Oncol 7 (2000): 657-662.
- Werther K, Christensen IJ, Nielsen HJ, et al. Prognostic impact of matched preoperative plasma and serum VEGF in patients with primary colorectal carcinoma. Br J Cancer 3 (2002): 417-423.
- Abdel-Gawad IA, Hassanein HM, Bahgat NA, et al. Study of Endothelin-1 and Vascular Endothelial Growth Factor in Patients with Cancer Colon. J Egypt Natl Canc Inst 3 (2008): 216-223.
- Co?kun Ö, Öztopuz Ö, Özkan ÖF. Determination of IL-6, TNF-alpha and VEGF levels in the serums of patients with colorectal cancer. Cell Mol Biol (Noisy-le-grand) 63 (2017): 97-101.
- Dbouk HA, Tawil A, Nasr F, et al. Significance of CEA and VEGF as Diagnostic Markers of Colorectal Cancer in Lebanese Patients. Open Clin Cancer J 1 (2007): 1-5.
- De Vita F, Orditura M, Lieto E, et al. Elevated Perioperative Serum Vascular Endothelial Growth Factor Levels in Patients with Colon Carcinoma. Cancer 100 (2003): 270-278.
- Karayiannakis AJ, Syrigos KN, Zbar A, et al. Clinical significance of preoperative serum vascular endothelial growth factor levels in patients with colorectal cancer and the effect of tumor surgery. Surgery 131 (2002): 548-555.
- Kushlinskii NE, Gershtein ES, Nikolaev AA, et al. Insulin-Like Growth Factors (IGF), IGF-Binding Proteins (IGFBP), and Vascular Endothelial Growth Factor (VEGF) in Blood Serum of Patients with Colorectal Cancer. Bull Exp Biol Med 156 (2014): 684-688.
- Spacek J, Vocka M, Netikova I, et al. Immunological examination of peripheral blood in patients with colorectal cancer compared to healthy controls. Immunol Invest (2018): 1-11.
- Corley DA, Peek RM. When Should Guidelines Change? A Clarion Call for Evidence Regarding the Benefits and Risks of Screening for Colorectal Cancer at Earlier Ages. Gastroenterology 155 (2018): 947-949.
- Zhang Z, Ji S, Zhang B, et al. Role of angiogenesis in pancreatic cancer biology and therapy. Biomed Pharmacother 108 (2018): 1135-1140.
- Li XG, Wang YX, Zhou BQ, et al. The effect of VEGF-ASODN transfection on expression of VEGF and growth in tongue squamous cancer cell line Tca8113. Shanghai Kou Qiang Yi Xue 18 (2009): 509-514.
- Liang X, Xu F, Li X, et al. VEGF signal system: the application of antiangiogenesis. Curr Med Chem 21 (2014): 894-910.
- Bottomley MJ, Webb NJ, Watson CJ, et al. Placenta growth factor (PlGF) induces vascular endothelial growth factor (VEGF) secretion from mononuclear cells and is co-expressed with VEGF in synovial fluid. Clin Exp Immunol 119 (2000): 182-188.
- Bhattacharya R, Fan F, Wang R, et al. Intracrine VEGF signalling mediates colorectal cancer cell migration and invasion. Br J Cancer 117 (2017): 848-855.
- Yamagishi N, Teshima-Kondo S, Masuda K, et al. Chronic inhibition of tumor cell-derived VEGF enhances the malignant phenotype of colorectal cancer cells. BMC Cancer 13 (2013): 229.
- Guo Q, Shengbin D, Shen F, et al. VEGF +405G/C (rs2010963) polymorphisms and digestive system cancer risk: a meta-analysis. Tumour Biol 35 (2014): 4977-4982.
- Levin B, Lieberman DA, McFarland B, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 134 (2008): 1570-1595.
