Assessment of Urinary Sediments in the Diagnosis of Different Types of Primary Glomerulonephritis
Article Information
Md. Rezaul Alam1*, Muhammad Nazrul Islam1, Ferdous Jahan1, Tofael Ahammod2, AKM Shahidur Rahman1
1Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
2Department of Nephrology, Shaheed Syed Nazrul Islam Medical College, Kishorgonj, Bangladesh
*Corresponding Author: Dr. Md. Rezaul Alam, Medical Officer, Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Received: 08December2022; Accepted: 15 December2022; Published: 24 December2022
Citation:
Alam MR, Islam MN, Jahan F, Ahammod T, Rahman AKMS. Assessment of Urinary Sediments in the Diagnosis of Different Types of Primary Glomerulonephritis. Archives of Nephrology and Urology 5 (2022): 64-72.
View / Download Pdf Share at FacebookAbstract
Background: Glomerulonephritis (GN) is one of the major causes of chronic kidney disease (CKD) throughout the world and is leading causes of end stage renal disease (ESRD). The cellular and acellular materials that are present in urine sample of GN patients have great clinical significance.
Objective: To assess urinary sediments in the diagnosis of different types of primary glomerulonephritis.
Methods: This study was conducted at Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh. A total of 100 adult patients with primary glomerular disease were enrolled in this study and their renal biopsy was done. Fresh midstream urine (MSU) sample (10 ml) was obtained from each patient and was analyzed accordingly.
Results: Mean(±SD) age of the study subjects was 34.5±14.0 years. Of them; 60% were male and 40% were female, male to female ratio was 1.5:1. Among 100 study participants 32(32.0%) were non-proliferative glomerulonephritis and 68(68.0%) were proliferative glomerulonephritis. It was observed that, hypertension was more common in proliferative group than non-proliferative group. Urinalysis revealed that; most of the varieties of proliferative glomerulonephritis presented with 2+ protein in
urine, less than 10/HPF of white blood cell (WBC) in urine, more than 10/ HPF red blood cells (RBC) and granular cast in urine along with occasional very few red blood cell cast (RBC cast) in urine. Among total cases; 55% presented with nephrotic range proteinuria. Renal failure was present in 54% cases and more marked in proliferative glomerulonephritis.
Conclusion: Urinary sediments analysis along with other clinical and biochemical parameters can help us to distinguish proliferative types of glomerulonephritis, although particular variety of glomerulonephritis is difficult to confirm by u
Keywords
Chronic Kidney Disease (CKD); Glomerulonephritis (GN); Hypertension; Renal failure; Urinary sediments
Chronic Kidney Disease (CKD) articles; Glomerulonephritis (GN) articles; Hypertension articles; Renal failure articles; Urinary sediments articles
Chronic Kidney Disease articles Chronic Kidney Disease Research articles Chronic Kidney Disease review articles Chronic Kidney Disease PubMed articles Chronic Kidney Disease PubMed Central articles Chronic Kidney Disease 2023 articles Chronic Kidney Disease 2024 articles Chronic Kidney Disease Scopus articles Chronic Kidney Disease impact factor journals Chronic Kidney Disease Scopus journals Chronic Kidney Disease PubMed journals Chronic Kidney Disease medical journals Chronic Kidney Disease free journals Chronic Kidney Disease best journals Chronic Kidney Disease top journals Chronic Kidney Disease free medical journals Chronic Kidney Disease famous journals Chronic Kidney Disease Google Scholar indexed journals Glomerulonephritis articles Glomerulonephritis Research articles Glomerulonephritis review articles Glomerulonephritis PubMed articles Glomerulonephritis PubMed Central articles Glomerulonephritis 2023 articles Glomerulonephritis 2024 articles Glomerulonephritis Scopus articles Glomerulonephritis impact factor journals Glomerulonephritis Scopus journals Glomerulonephritis PubMed journals Glomerulonephritis medical journals Glomerulonephritis free journals Glomerulonephritis best journals Glomerulonephritis top journals Glomerulonephritis free medical journals Glomerulonephritis famous journals Glomerulonephritis Google Scholar indexed journals Hypertension articles Hypertension Research articles Hypertension review articles Hypertension PubMed articles Hypertension PubMed Central articles Hypertension 2023 articles Hypertension 2024 articles Hypertension Scopus articles Hypertension impact factor journals Hypertension Scopus journals Hypertension PubMed journals Hypertension medical journals Hypertension free journals Hypertension best journals Hypertension top journals Hypertension free medical journals Hypertension famous journals Hypertension Google Scholar indexed journals Renal failure articles Renal failure Research articles Renal failure review articles Renal failure PubMed articles Renal failure PubMed Central articles Renal failure 2023 articles Renal failure 2024 articles Renal failure Scopus articles Renal failure impact factor journals Renal failure Scopus journals Renal failure PubMed journals Renal failure medical journals Renal failure free journals Renal failure best journals Renal failure top journals Renal failure free medical journals Renal failure famous journals Renal failure Google Scholar indexed journals Urinary sediments articles Urinary sediments Research articles Urinary sediments review articles Urinary sediments PubMed articles Urinary sediments PubMed Central articles Urinary sediments 2023 articles Urinary sediments 2024 articles Urinary sediments Scopus articles Urinary sediments impact factor journals Urinary sediments Scopus journals Urinary sediments PubMed journals Urinary sediments medical journals Urinary sediments free journals Urinary sediments best journals Urinary sediments top journals Urinary sediments free medical journals Urinary sediments famous journals Urinary sediments Google Scholar indexed journals minimal change diseases articles minimal change diseases Research articles minimal change diseases review articles minimal change diseases PubMed articles minimal change diseases PubMed Central articles minimal change diseases 2023 articles minimal change diseases 2024 articles minimal change diseases Scopus articles minimal change diseases impact factor journals minimal change diseases Scopus journals minimal change diseases PubMed journals minimal change diseases medical journals minimal change diseases free journals minimal change diseases best journals minimal change diseases top journals minimal change diseases free medical journals minimal change diseases famous journals minimal change diseases Google Scholar indexed journals Red Blood Cell articles Red Blood Cell Research articles Red Blood Cell review articles Red Blood Cell PubMed articles Red Blood Cell PubMed Central articles Red Blood Cell 2023 articles Red Blood Cell 2024 articles Red Blood Cell Scopus articles Red Blood Cell impact factor journals Red Blood Cell Scopus journals Red Blood Cell PubMed journals Red Blood Cell medical journals Red Blood Cell free journals Red Blood Cell best journals Red Blood Cell top journals Red Blood Cell free medical journals Red Blood Cell famous journals Red Blood Cell Google Scholar indexed journals Focal and segmental glomerulosclerosis articles Focal and segmental glomerulosclerosis Research articles Focal and segmental glomerulosclerosis review articles Focal and segmental glomerulosclerosis PubMed articles Focal and segmental glomerulosclerosis PubMed Central articles Focal and segmental glomerulosclerosis 2023 articles Focal and segmental glomerulosclerosis 2024 articles Focal and segmental glomerulosclerosis Scopus articles Focal and segmental glomerulosclerosis impact factor journals Focal and segmental glomerulosclerosis Scopus journals Focal and segmental glomerulosclerosis PubMed journals Focal and segmental glomerulosclerosis medical journals Focal and segmental glomerulosclerosis free journals Focal and segmental glomerulosclerosis best journals Focal and segmental glomerulosclerosis top journals Focal and segmental glomerulosclerosis free medical journals Focal and segmental glomerulosclerosis famous journals Focal and segmental glomerulosclerosis Google Scholar indexed journals
Article Details
1. Introduction
Glomerulonephritis (GN) is an immune complex mediated disease characterized by the deposition of immune reactants particularly immunoglobulins and complements in the glomerulus; which is accompanied by varying degrees of glomerular injury presented with nephrotic syndrome, nephritic illness, proteinuria, haematuria, rapidlyprogressiveglomerulonephritis (RPGN) etc. [1]. Glomerulonephritis (GN) are group of diseases of inflammatory or non-inflammatory nature involving the renal glomeruli [2]. There is a wide variety of disorders which affect the glomeruli, the basic filtering units of the kidney [2]. This can occur either as a primary glomerular diseases or secondary to any cause like infection, drugs or tumor [2,3]. Glomerulonephritis may be isolated or a manifestation of renal involvement in a systemic disease [4]. Any age group may be affected, although some types are particularly common in children e.g. post streptococcal glomerulonephritis, minimal change diseases (MCD) [5]. Glomerulonephritis is one of the major causes of chronic kidney disease (CKD) which is the leading cause of end stage renal disease (ESRD) and is an important cause of morbidity and mortality throughout the world [6]. Spectrum of glomerular diseases varies significantly in different parts of the world as it is influenced by geographical, environmental and socioeconomic factors [6]. Injury to glomeruli results in a variety of signs and symptoms of disease including;oedema, hypertension, oliguria, proteinuria, haematuria, azotemia etc. [4]. Specific glomerular diseases tend to produce relatively particular syndrome of renal dysfunction, although different varieties of glomerular diseases may produce the same syndrome [2,3]. Clinical diagnosis of specific glomerular diseases is difficult, because the same glomerular disease can manifest in different ways, due to the great variations of clinical manifestations of glomerular disease, therefore diagnosis depends on clinical features, laboratory data and histological analysis [2,3]. Histological analysis of renal biopsy remains the gold standard for diagnosing glomerulonephritis [7]. Histological analysis of renal biopsy plays a fundamental role in the evaluation of patients with haematuria and/or proteinuria [7]. The findings of histopathology report helps in accurate diagnosis of diseases, selecting appropriate treatment with assessment of prognosis of diseases [8,9]. Although renal biopsy is an invasive procedure and not well-accepted by all patients, moreover it needs hospitalization [10]. Primary glomerular diseases present with edema, often with hypertension, oliguria, abnormalities in the urine and impaired renal function [11,12]. Urinary abnormalities include- proteinuria, haematuria, presence of cast etc. are often present in the urine of patients with glomerular diseases [10,13]. Cellular casts are particularly significant because it indicate acute inflammation of the kidney [14]. In clinical practice, clinician needs a relatively simple, affordable technique like urinary sediments examination that may help to diagnose different types of primary glomerular diseases. In this study we tried to correlate urinary sediments with primary glomerulonephritis in adult population.
2. Methodology
This cross sectional study was conducted at the Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh. The study protocol was approved by the Institutional Review Board (IRB) of BSMMU, Dhaka, Bangladesh. A total of one hundred (100) clinically diagnosed patients of glomerulonephritis were selected by purposive sampling technique following selection criteria. After taking informed written consent all study patients were evaluated by detailed history, relevant clinical examination and routine laboratory investigations. Adult patients (age 18 years to 60 years), clinically suspected primary glomerulonephritis with sonographically normal size kidney (kidney size: 9-11cm) were included. Patients with established non-glomerular haematuria [Renal stone, urothelial malignancy, renal tuberculosis, renal cysts, urinary tract infection (UTI) etc.], all secondary glomerulonephritis (Lupus nephritis, diabetic nephropathy, vasculitis etc.) and patients having any bleeding disorders were excluded from the study
2.1 Grouping of the study patients
A total of 100 adult patients presenting with clinical and laboratory features suggestive of primary glomerular disease enrolled and their renal biopsy was done following standard procedure. Then all study patients were subdivided into 2 groups- group A: Non-proliferative glomerulonephritis and group B: proliferative glomerulonephritis. Patients in Group A were further subdivided into 3 subgroups; A1 - Minimal change disease (MCD), A2 - Focal and segmental glomerulosclerosis and A3 - Membranous glomerulonephritis. Patients in Group B also further subdivided into 4 subgroups; B1 - Mesangial proliferative glomerulonephritis, B2 - IgA nephropathy, B3 –Mesangio-capillary glomerulonephritis, B4 - Chronic sclerosing glomerulonephritis.
2.2. Urinary sediments
It may be defined as the cellular and acellular materials that are present in centrifuged urine sample of great clinical significance; e.g., cells [Red blood cell (RBC), white blood cell (WBC)], casts (RBC casts, WBC casts, and granular casts). Microscopic examination of urine in conjunction with dipstick chemical analysis aids in the detection of renal and urinary tract disease process.
Casts: These are plugs of Tamm-Horsfallmucoprotein within the renal tubules, conferring a characteristic cylindrical or tubular shape. They are classified according to appearance and the cellular elements embedded in them: non-celluar/acelluar casts (Hyaline cast, granular cast, waxy cast) and cellular casts [Red cell cast (RBC cast), white cell cast (WBC cast), epithelial cell cast, fatty cast].
2.3. Collection and analysis of urine samples
Fresh midstream urine (MSU) sample (10ml) was obtained from each patient in a clean and dry test tube with proper labeling, then immediately all samples were sent to kidney research laboratory, Department of Nephrology, BSMMU. Urine analysis was done within two hours after collection of samples.
Dipstick strip test: The dipstick strip was used for the detection of protein and glucose in urine.
Microscopic examination of urine: For microscopic examination of urine sediment, 5ml of urine was centrifuge in a conical tube for 10 minutes at 2000 rpm. After centrifugation, the supernatant was discarded and resuspend the sediment in a few drops (about 0.5ml). Then remaining urine was mixed by flicking the end of the tube with the finger. One drop of well mixed sediment was placed on a clean glass slides and covered with a cover slip measuring 18mm×18mm. This slide was then examined under high power objective for urine sediment observation.
2.4. Data collection and analysis
All necessary and relevant data of the study patients were recorded methodically in a data collection sheet. All the cases were numbered chronologically. Same number was given to slide and biopsy reports. Statistical analysis of data was performed using computer based software program Statistical Packages for Social Sciences 22 (SPSS- 22). All quantitative data were expressed as mean and standard deviation (SD), while frequency (n) and percentage (%) was used for qualitative data. The statistics used to analyze the data was simple descriptive statistics. Comparison of continuous variables was done by unpaired‘t’ test and chi-square test. A p value <0.05 was considered as significant.
3. Results and Observations
This study was intended to assess urinary sediments in the diagnosis of different types of primary glomerulonephritis (GN). A total of 100 patients with primary glomerulonephritis were selected accordingly. The mean(±SD) age of the study patients was 34.5±14.0 years and ranged from 18 years to 60 years. Of them; 21% was below the age of 20 years, 31% was in the age group of 21-30 years, 27% was in the age group of 31-40 years and 21% was above the age of 40 years (Figure- 1). Among the study subjects; 60% was male and rest 40% was female (Figure- 2). The study patients were divided into 2 groups- group A [Non-proliferative glomerulonephritis (n=32; male- 20, female- 12)] and group B [Proliferative glomerulonephritis (n=68; male- 40, female- 28)] (Table-1). Histological findings of renal biopsy revealed that 68% of primary glomerulonephritis was proliferative; of which mesangialproliferative glomerulonephritis (MsPGN) was 50%, mesangio-capillary glomerulonephritis (MCGN/MPGN) was 33.8%, IgA nephropathy was 11.8%, chronic sclerosing glomerulonephritis was 4.4%. Among non-proliferatrive glomerulonephritis (32); focal and segmental glomerulosclerosis (FSGS) was 59.4%, minimal change disease (MCD) was 25%, and membranous glomerulonephritis (MGN) was 15.6% (Table- 2). It was observed that majority (31.3%) patients in group A was above 40 years and majority (33.8%) patients in group B was between 21-30 years, but there was no significant age difference between the groups (p= 0.217) (Table-3). Patients of Group A were further sub-divided into 3 subgroups; A1- Minimal change disease, A2- Focal and segmental glomerulosclerosisand A3- Membranous glomerulonephritis. Similarly patients of Group B were also further subdivided into 4 sub-groups; B1- Mesangial proliferative glomerulonephritis, B2- IgA nephropathy, B3- Mesangio-capillary glomerulonephritis, B4 - Chronic sclerosing glomerulonephritis (Table-4). Table- 4 displayingthe distribution of study patients according to the age in different subgroups. Male predominance was observed in different subgroups (Table-5).
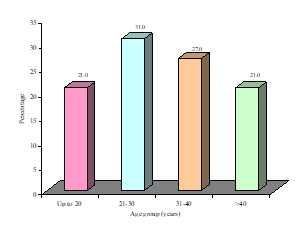
Figure-1: Overall age distribution of the study patients (N=100)
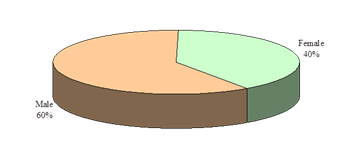
Figure-2: Overall gender distribution of the study patients (N=100)
|
Groups |
Male No. (%) |
Female No. (%) |
p value |
|
A (n=32) |
20(62.5) |
12(37.5) |
0.726ns |
|
B (n=68) |
40(58.8) |
28(41.2) |
Group A: Non-proliferativeglomerulonephritis, Group B: Proliferativeglomerulonephritis, Chi square test was done, ns= Notsignificant
Table- 1: Group and gender distribution of study participants (N= 100)
|
Histopathological diagnosis |
Number (n) |
Percentage (%) |
|
Proliferative glomerulonephritis (n=68) |
||
|
Ms PGN |
34 |
50 |
|
MPGN |
23 |
33.8 |
|
IgA Nephropathy |
8 |
11.8 |
|
Chronic sclerosing GN |
3 |
4.4 |
|
Non-proliferative glomerulonephritis (n=32) |
||
|
FSGS |
19 |
59.4 |
|
MCD |
8 |
25 |
|
MGN |
5 |
15.6 |
MsPGN= Messangial Proliferative Glomerulonephritis, MPGN= Membranoproliferative Glomerulonephritis, IgA Nephropathy= Immunoglobulin A Nephropathy, Chronic sclerosing GN= Chronic Sclerosing Glomerulonephritis, FSGS= Focal and Segmental Glomerulosclerosis, MCD= Minimal Change Disease, MGN= Membranous Glomerulonephritis
Table- 2: Distribution of study population according to the histopathological pattern of glomerulonephritis (N=100)
|
Groups |
Age (years) |
p value |
|||
|
≤20 No. (%) |
21-30 No. (%) |
31-40 No. (%) |
>40 No. (%) |
||
|
A (n=32) |
8(25.0) |
8(25.0) |
6(18.8) |
10(31.3) |
0.217ns |
|
B (n=68) |
13(19.1) |
23(33.8) |
21(30.9) |
11(16.2) |
|
Group A: Non-proliferative Glomerulonephritis, Group B: Proliferative Glomerulonephritis, Chi square test was done, ns = Not significant
Table-3: Group wise age distribution of the study patients (N= 100)
|
Subgroups |
Age (years) |
|||
|
≤20 No. (%) |
21-30 No. (%) |
31-40 No. (%) |
>40 No. (%) |
|
|
A1 (n=8) |
5(62.5) |
3(37.5) |
0 |
0 |
|
A2 (n=19) |
3(15.8) |
5(26.3) |
4(21.1) |
7(36.8) |
|
A3 (n=5) |
0 |
0 |
2(40.0) |
3(60.0) |
|
B1 (n=34) |
6(17.6) |
12(35.3) |
9(26.5) |
7(20.6) |
|
B2 (n=8) |
3(37.5) |
2(25.0) |
3(37.5) |
0 |
|
B3 (n=23) |
4(17.4) |
8(34.8) |
8(34.8) |
3(13.0) |
|
B4 (n=3) |
0 |
1(33.3) |
1(33.3) |
1(33.3) |
Subgroup A1: Minimal change disease, Subgroup A2: Focal and segmental glomerulosclerosis, Subgroup A3: Membranous glomerulonephritis, Subgroup B1: Mesangial proliferative glomerulonephritis, Subgroup B2: IgA nephropathy, Subgroup B3: Mesangio-capillary glomerulonephritis, Subgroup B4: Chronic sclerosing glomerulonephritis
Table- 4: Different subgroup wise age distribution of the study patients (N= 100)
|
Subgroups |
Male No. (%) |
Female No. (%) |
|
A1 (n=8) |
7(87.5) |
1(12.5) |
|
A2 (n=19) |
9(47.4) |
10(52.6) |
|
A3 (n=5) |
4(80.0) |
1(20.0) |
|
B1 (n=34) |
19(55.9) |
15(44.1) |
|
B2 (n=8) |
3(37.5) |
5(62.5) |
|
B3 (n=23) |
16(69.6) |
7(30.4) |
|
B4 (n=3) |
2(66.7) |
1(33.3) |
A1: Minimal change disease, A2: Focal and segmental glomerulosclerosis, A3: Membranous glomerulonephritis, B1: Mesangial proliferative glomerulonephritis, B2: IgA nephropathy, B3: Mesangio-capillary glomerulonephritis, B4: Chronic sclerosing glomerulonephritis
Table-5: Subgroup wise gender distribution of the study patients (N= 100)
Data analysis revealed that, half (50%) of the patients of group A had hypertension and 45.6% patients of group B was hypertensive (p= 0.680) (Table-6). Table-7 showing the distribution of study patients on the basis of hypertension in different subgroups.
|
Groups |
Hypertension present No. (%) |
Hypertension absent No. (%) |
p value |
|
A (n=32) |
16(50.0) |
16(50.0) |
0.680ns |
|
B (n=68) |
31(45.6) |
37(54.4) |
Group A: Non-proliferative glomerulonephritis, Group B: Proliferative glomerulonephritis, Chi square test was done, ns = Not significant
Table- 6: Group wise status of hypertension among the study patients (N= 100)
|
Subgroups |
Hypertension present No. (%) |
Hypertension absent No. (%) |
|
A1 (n=8) |
0 |
8(100.0) |
|
A2 (n=19) |
13(68.4) |
6(31.6) |
|
A3 (n=5) |
3(60.0) |
2(40.0) |
|
B1 (n=34) |
7(20.6) |
27(79.4) |
|
B2 (n=8) |
4(50.0) |
4(50.0) |
|
B3 (n=23) |
17(73.9) |
6(26.1) |
|
B4 (n=3) |
3(100.0) |
0 |
A1: Minimal change disease, A2: Focal and segmental glomerulosclerosis, A3: Membranous glomerulonephritis, B1: Mesangial proliferative glomerulonephritis, B2: IgA nephropathy, B3: Mesangio-capillary glomerulonephritis, B4: Chronic sclerosing glomerulonephritis
Table-7: Subgroup wise status of hypertension among the study patients (N= 100)
In this study, proteinuria was not significantly different between group A and group B patients (p=0.607) (Table-8). Although, patients with proteinuria (2++ and 3+++) were frequently observed in different sub groups (Table-9).
|
Groups |
Urinary protein |
p value |
|
|
2++ No. (%) |
3+++ No. (%) |
||
|
A (n=32) |
19(59.4) |
13(40.6) |
0.607ns |
|
B (n=68) |
44(64.7) |
24(35.3) |
|
Group A: Non-proliferative glomerulonephritis, Group B: Proliferative glomerulonephritis, Chi square test was done, ns = Not significant
Table-8: Group wise status of urinary protein among the study patients (N=100)
|
Subgroups |
Urinary protein |
|
|
2++ No. (%) |
3+++ No. (%) |
|
|
A1 (n=8) |
2(25.0) |
6(75.0) |
|
A2 (n=19) |
13(68.4) |
6(31.6) |
|
A3 (n=5) |
4(80.0) |
1(20.0) |
|
B1 (n=34) |
23(67.6) |
11(32.4) |
|
B2 (n=8) |
6(75.0) |
2(25.0) |
|
B3 (n=23) |
14(60.9) |
9(39.1) |
|
B4 (n=3) |
1(33.3) |
2(66.7) |
A1: Minimal change disease, A2: Focal and segmental glomerulosclerosis, A3: Membranous glomerulonephritis, B1: Mesangial proliferative glomerulonephritis, B2: IgA nephropathy, B3: Mesangio-capillary glomerulonephritis, B4: Chronic sclerosing glomerulonephritis
Table-9:Subgroup wise status of urinary protein among the study patients (N= 100)
White blood cell (WBC) in urine was not significantly (p=0.779) different between the groups (group A and group B) (Table- 10). While, leucocytes (WBC) were more frequently found in urine samples among patients in group B (Table- 11).
|
Groups |
white blood cells (WBC) |
p value |
|
|
≤10/HPF No. (%) |
>10/HPF No. (%) |
||
|
A (n=32) |
23(71.9) |
9(28.1) |
0.779ns |
|
B (n=68) |
47(69.1) |
21(30.9) |
|
Group A: Non-proliferative glomerulonephritis, Group B: Proliferative glomerulonephritis, Chi square test was done, ns = Not significant
Table-10: Group wise status of white blood cells (WBC) in urine among the study patients (N= 100)
|
Subgroups |
White Blood Cell (WBC) |
|
|
≤10/HPF No. (%) |
>10/HPF No. (%) |
|
|
A1 (n=8) |
6(75.0) |
2(25.0) |
|
A2 (n=19) |
12(63.2) |
7(36.8) |
|
A3 (n=5) |
5(100.0) |
0 |
|
B1 (n=34) |
23(67.6) |
11(32.4) |
|
B2 (n=8) |
8(100.0) |
0 |
|
B3 (n=23) |
14(60.9) |
9(39.1) |
|
B4 (n=3) |
2(66.7) |
1(33.3) |
A1: Minimal change disease, A2: Focal and segmental glomerulosclerosis, A3: Membranous glomerulonephritis, B1: Mesangial proliferative glomerulonephritis, B2: IgA nephropathy, B3: Mesangio-capillary glomerulonephritis, B4: Chronic sclerosing glomerulonephritis
Table- 11: Subgroup wise status of WhiteBlood Cell(WBC) in urine among the study patients (N= 100)
Red Blood Cell (RBC) in urine was significantly different between group A and group B patients(p=0.0001) (Table-12). Although erythrocytes (RBC) were frequently found in urine samples among different sub groups (Table-13).
|
Groups |
Red Blood Cell (RBC) |
p value |
|
|
≤10/HPF No. (%) |
>10/HPF No. (%) |
||
|
A (n=32) |
31(96.9) |
1(3.1) |
0.0001s |
|
B (n=68) |
18(26.5) |
50(73.5) |
|
Group A: Nonproliferative glomerulonephritis, Group B: Proliferative glomerulonephritis, Chi square test was done, s= Significant
Table- 12: Group wise status of Red Blood Cell (RBC) in urine among the study patients (N= 100)
|
Subgrupos |
Red BloodCell (RBC) |
|
|
≤10/HPF No. (%) |
>10/HPF No. (%) |
|
|
A1 (n=8) |
8(100.0) |
0 |
|
A2 (n=19) |
18(94.7) |
1(5.3) |
|
A3 (n=5) |
5(100.0) |
0 |
|
B1 (n=34) |
11(32.4) |
23(67.6) |
|
B2 (n=8) |
0 |
8(100.0) |
|
B3 (n=23) |
6(26.1) |
17(73.9) |
|
B4 (n=3) |
1(33.3) |
2(66.7) |
A1: Minimal change disease, A2: Focal and segmental glomerulosclerosis, A3: Membranous glomerulonephritis, B1: Mesangial proliferative glomerulonephritis, B2: IgA nephropathy, B3: Mesangio-capillary glomerulonephritis, B4: Chronic sclerosing glomerulonephritis
Table-13: Subgroup wise status of Red Blood Cell (RBC) in urine among the study patients (N= 100)
Red Blood Cell cast (RBC cast) in urine was not significantly different between group A and group B (p=0.431) (Table- 14). However RBC cast was occasionally seen in urine samples among different sub groups (Table-15).
|
Groups |
Red Blood Cell Cast (RBC cast) |
p value |
|
|
Present No. (%) |
Absent No. (%) |
||
|
A (n=32) |
2(6.3) |
30(93.8) |
0.431ns |
|
B (n=68) |
2(2.9) |
66(97.1) |
|
Group A: Non-proliferative glomerulonephritis, Group B: Proliferative glomerulonephritis, Chi square test was done, ns= Not significant
Table-14: Group wise status of Red Blood Cell cast (RBC cast) in urine among the study patients (N= 100)
|
Subgroups |
Red Blood Cell Cast (RBC cast) |
|
|
Present No. (%) |
Absent No. (%) |
|
|
A1 (n=8) |
0 |
8(100.0) |
|
A2 (n=19) |
2(10.5) |
17(89.5) |
|
A3 (n=5) |
0 |
5(100.0) |
|
B1 (n=34) |
1(2.9) |
33(97.1) |
|
B2 (n=8) |
1(12.5) |
7(87.5) |
|
B3 (n=23) |
0 |
23(100.0) |
|
B4 (n=3) |
0 |
3(100.0) |
A1: Minimal change disease, A2: Focal and segmental glomerulosclerosis, A3: Membranous glomerulonephritis, B1: Mesangial proliferative glomerulonephritis, B2: IgA nephropathy, B3: Mesangio-capillary glomerulonephritis, B4: Chronic sclerosing glomerulonephritis
Table-15: Subgroup wise status of Red Blood Cell Cast (RBC cast) in urine among the study patients (N= 100)
Granular cast in urine was more frequently observed in proliferative glomerulonephritis cases than non-proliferative glomerulonephritis cases, but that was not significantly different between the groups (48.5% versus 31.3%,p=0.103) (Table- 16). But granular cast was frequently found in urine samples among different sub groups (Table-17).
|
Groups |
Granular casts |
p value |
|
|
Present No. (%) |
Absent No. (%) |
||
|
A (n=32) |
10(31.3) |
22(68.8) |
0.103ns |
|
B (n=68) |
33(48.5) |
35(51.5) |
|
Group A: Non-proliferative glomerulonephritis, Group B: Proliferative glomerulonephritis, Chi square test was done, ns= Not significant
Table-16: Group wise status of granular cast in urine among the study patients (N= 100)
|
Subgroups |
Granular casts |
|
|
Present No. (%) |
Absent No. (%) |
|
|
A1 (n=8) |
1(12.5) |
7(87.5) |
|
A2 (n=19) |
9(47.4) |
10(52.6) |
|
A3 (n=5) |
0 |
5(100.0) |
|
B1 (n=34) |
19(55.9) |
15(44.1) |
|
B2 (n=8) |
2(25.0) |
6(75.0) |
|
B3 (n=23) |
10(43.5) |
13(56.5) |
|
B4 (n=3) |
2(66.7) |
1(33.3) |
A1: Minimal change disease, A2: Focal and segmental glomerulosclerosis, A3: Membranous glomerulonephritis, B1: Mesangial proliferative glomerulonephritis, B2: IgA nephropathy, B3: Mesangio-capillary glomerulonephritis, B4: Chronic sclerosing glomerulonephritis
Table-17: Subgroup wise status of granular cast among the study patients (N= 100)
It was observed that patients with massive proteinuria [Urinary Total Protein (UTP) ≥3.5 gm/24hours] were significantly frequent in group A than group B (p=0.001) (Table- 18). In this study nephritic/nephrotic range proteinuria was frequently observed in different sub groups (Table- 19).
|
Groups |
UrinaryTotal Protein (UTP) |
p value |
|
|
<3.5 g/24h No. (%) |
≥3.5 g/24h No. (%) |
||
|
A (n=32) |
7(21.9) |
25(78.1) |
0.001s |
|
B (n=68) |
38(55.9) |
30(44.1) |
|
Group A: Non-proliferative glomerulonephritis, Group B: Proliferative glomerulonephritis, Chi square test was done, s= significant
Table-18: Group wise status of urinary total protein (UTP) among the study patients (N= 100)
|
Subgroups |
Urinary total protein (UTP) |
|
|
<3.5 g/24h No.(%) |
≥3.5 g/24h No.(%) |
|
|
A1 (n=8) |
1(12.5) |
7(87.5) |
|
A2 (n=19) |
6(31.6) |
13(68.4) |
|
A3 (n=5) |
0 |
5(100.0) |
|
B1 (n=34) |
17(50.0) |
17(50.0) |
|
B2 (n=8) |
7(87.5) |
1(12.5) |
|
B3 (n=23) |
12(52.2) |
11(47.8) |
|
B4 (n=3) |
2(66.7) |
1(33.3) |
A1: Minimal change disease, A2: Focal and segmental glomerulosclerosis, A3: Membranous glomerulonephritis, B1: Mesangial proliferative glomerulonephritis, B2: IgA nephropathy, B3: Mesangio-capillary glomerulonephritis, B4: Chronic sclerosing glomerulonephritis
Table- 19: Subgroup wise status of Urinary Total Protein (UTV) among the study patients (N= 100)
It was found that patients with raised serum creatinine level were significantly frequent in group B than group A (p=0.007) (Table-20). In this series, serum creatinine level was comparatively higher among patients in different sub groups of group B than that of group A (Table- 21).
|
Groups |
Serum Creatinine level |
p value |
|
|
<120 μmol/L No. (%) |
≥120 μmol/L No. (%) |
||
|
A (n=32) |
21(65.6) |
11(34.4) |
0.007s |
|
B (n=68) |
25(36.8) |
43(63.2) |
|
Group A: Non-proliferative glomerulonephritis, Group B: Proliferative glomerulonephritis, Chi square test was done, s= significant
Table-20: Group wise status of serum creatinine level of the study patients (N= 100)
|
Subgroups |
Serum creatinine level |
|
|
<120 μmol/L No. (%) |
≥120 μmol/L No. (%) |
|
|
A1 (n=8) |
8(100.0) |
0 |
|
A2 (n=19) |
9(47.4) |
10(52.6) |
|
A3 (n=5) |
4(80.0) |
1(20.0) |
|
B1 (n=34) |
16(47.1) |
18(52.9) |
|
B2 (n=8) |
4(50.0) |
4(50.0) |
|
B3 (n=23) |
5(21.7) |
18(78.3) |
|
B4 (n=3) |
0 |
3(100.0) |
A1: Minimal change disease, A2: Focal and segmental glomerulosclerosis, A3: Membranous glomerulonephritis, B1: Mesangial proliferative glomerulonephritis, B2: IgA nephropathy, B3: Mesangio-capillary glomerulonephritis, B4: Chronic sclerosing glomerulonephritis
Table- 21: Subgroup wise status of serum creatinine level among the study patients (N=100)
4.Discussion
Glomerulonephritis (GN) continue to be leading cause of End Stage Renal Disease (ESRD) in developing countries particularly in Bangladesh [15]. Among the different causes of ESRD, glomerulonephritis comprises 47% in Bangladesh [16]. Hence, it is important to recognize the pattern of glomerulonephritis in any given geographical area as pattern of glomerulonephritis are changing with time [2,17]. Glomerular diseases generally present with nephrotic syndrome, nephritic syndrome, variable degree of proteinuria with or without haematuria (Microscopic or macroscopic) and impaired renal function [3,4,8-14,18]. As an aid to diagnosis of glomerular disease, assessment of urinary sediment may play an important role in different types of primary glomerulonephritis [8-14]. This study was aimed to assess urinary sediments in the diagnosis of different types of primary glomerulonephritis.
We evaluated 100 adult patients presenting with clinical and laboratory features suggestive of primary glomerular disease and their renal biopsy was done following standard procedure. The mean age of the study patients was 34.5±14.0 years and the lowest and highest ages were 18 years and 60 years respectively. Age distribution of the study subjects showed that, out of 100 study patients; 21% was < 20 years, 31% patients were in 21-30 years age group, 27% patients in 31-40 years age group and 21% patients were more than 40 years old. Out of 100 study patients; 60% were male and 40% were female, with a male to female ratio was 1.5:1. It was reported that males are affected more than females with primary glomerular disease in general [9]. The patients were subdivided into 2 groups; group A – non-proliferative glomerulonephritis and group B – proliferative glomerulonephritis. Patients in Group A were further divided into 3 subgroups; A1- Minimal change disease. A2- Focal and segmental glomerulosclerosis, and A3- Membranous glomerulonephritis. Similarly patients in Group B were also divided into 4 subgroups; B1- Mesangial proliferative glomerulonephritis, B2- IgA nephropathy, B3- Mesangio-capillary glomerulonephritis, B4- Chronic sclerosing glomerulonephritis.
Among 100 study patients we found 32(32.0%) cases were in non-proliferative glomerulonephritis group and 68(68.0%) cases were proliferative glomerulonephritis. In the proliferative glomerulonephritis group out of 68 cases; we found 34 cases were mesangial proliferative glomerulonephritis, 23 cases were mesangio-capillary glomerulonephritis, 8 cases were IgA nephropathy and 3 cases were chronic sclerosing glomerulonephritis. This finding was consistent with related previous studies [9,19-21]. Among non-proliferative glomerulonephritis out of 32 cases; focal and segmental glomeruloscrelorosis was 59.4%, minimal change disease (MCD) was 25% and membranous glomerulonephritis was 15.6%. This result was also in a line of similar previous studies [19,22-24].
In this study among non-proliferative GN group; hypertension was present in 68.4% of focal and segmental glomerulosclerosis (FSGS) and 60% of membranous glomerulonephritis but hypertension was absent in 100% of minimal change disease (MCD). Khanam et al. showed that hypertension was present in 24% of focal and segmental glomerulosclerosis, 41% of membranous glomerulonephritis and 3% of minimal change disease (MCD) [19]. Although, it is believed that minimal change disease (MCD) does not present with hypertension [25]. In this present study, among proliferative GN group, hypertension was present in 73.9% of mesangio-capillary glomerulonephritis, 50% of IgA nephropathy, 20.6% of mesangial proliferative glomerulonephritis and 100% of chronic sclerosing glomerulonephritis, which was nearly similar to the findings of a related previous study [19]. Therefore, hypertension was more common in proliferative group than non-proliferative group.
Status of urinary protein among non-proliferative group showed that, 62.5% of minimal change disease (MCD) and 31.6% of focal and segmental glomerulosclerosis presented with 3+ proteinuria, and 25% of minimal change disease (MCD) and 68.4% of focal and segmental glomerulosclerosis (FSGS) and 80% of membranous glomerulonephritis were presented with 2+ proteinuria. Among proliferative glomerulonephritis group it was observed that; 61.8% of mesangial proliferative glomerulonephritis, 75% of IgA nephropathy, 60.9% of mesangio-capillary glomerulonephritis and 33.3% of chronic sclerosing glomerulonephritis presented with 2+ proteinuria. On the other hand, most of the non-proliferative glomerulonephritis was presented with 3+ proteinuria. These findings were comparable with a couple of previous studies [5,8,10]. In non-proliferative glomerulonephritis urinalysis showed that; 75% of minimal change disease (MCD), 63.2% of focal and segmental glomerulosclerosis (FSGS) and 100% of membranous glomerulonephritis were presented with <10/HPF of white blood cell (WBC)in urine. While in proliferative glomerulonephritis; 67.6% of mesangial proliferative glomerulonephritis, 100% of IgA nephropathy and 60.9% of mesangio-capillary glomerulonephritis were presented with <10/HPF of WBC in urine. It was also found that; 32.4% of mesangial proliferative glomerulonephritis, 39.1% of mesangio-capillary glomerulonephritis and 33.3% of chronic sclerosing glomerulonephritis presented with >10/HPF WBC in urine. Urinalysis showed that, out of 100 study cases 96 cases were presented with red blood cell (RBC) in urine. Among them 67.6% of mesangial proliferative glomerulonephritis, 73.9% of mesangio-capillary glomerulonephritis and 66.7% of chronic sclerosing glomerulonephritis presented with >10/HPF RBC in urine. On the other hand in non-proliferative glomerulonephritis group; 62.6% of minimal change disease (MCD), 36.8% of focal and segmental glomerulosclerosis (FSGS) and 80% of membranous glomerulonephritis were presented with <5/HPF RBC in urine. It was revealed that out of 100 studycases; RBC cast was present in 4 cases. Only 12.5% of IgA nephropathy, 10.5% of focal and segmental glomerulosclerosis (FSGS) and 2.9% of mesangial proliferative glomerulonephritis cases showed RBC cast in urine. While 97.0% of mesangial proliferative glomerulonephritis, 87.5% of IgA nephropathy and 89.5% of focal and segmental glomerulosclerosis (FSGS) cases showed no RBC cast in urine. On the other hand, RBC cast was absent in 100% cases of minimal change disease (MCD), 89.5% cases of focal and segmental glomerulosclerosis (FSGS) and 100% cases of membranous glomerulonephritis. It was observed that out of 100 study cases; granular cast was present in 43 cases. Among proliferative glomerulonephritis group granular casts were present in 56% of mesangial proliferative glomerulonephritis, 43.4% of msengio-capillary glomerulonephritis, 66.7% of chronic sclerosing glomerulonephritis and 25.0% of IgA nephropathy cases. But in non-proliferative glomerulonephritis group; granular casts were absent in 87.5% of minimal change disease (MCD), 52.6% of focal and segmental glomerulosclerosis (FSGS) and 100% membranous glomerulonephritis cases. These findings were consistent with similar previous studies [10-14].
In this present study 55% cases was presented with nephrotic range proteinuria and rest 45.0% cases was presented with non-nephrotic range proteinuria. A previous study found that 52% patients were presented with nephrotic range proteinuria and 42% was presented with non-nephrotic range proteinuria, which was almost similar to this present study [19]. It was observed that among non- proliferative group; 100% of membranous glomerulonephritis, 87.5% of minimal change disease (MCD) and 68.4% of focal and segmental glomerulosclerosis (FSGS) patients were presented with nephrotic range proteinuria. On the other hand among proliferative glomerulonephritis group; 50% of mesangial proliferative glomerulonephritis, 87.5% of IgA nephropathy, 52.2% of mesangio-capillary glomerulonephritis and 66.7% of chronic sclerosing glomerulonephritis cases were presented with non-nephrotic range proteinuria. These results were supported by related previous studies [3,5,8]. In this current study among proliferative glomerulonephritis group; 50% of mesangial proliferative glomerulonephritis, 47.8% of masangio-capillary glomerulonephritis, 33.3% of chronic sclerosing glomerulonephritis and 12.5% of IgA nephropathy cases were presented with nephrotic range proteinuria. In this series Khanam et al. showed that 61% of minimal change disease (MCD), 51% of membranous glomerulonephritis, 53% of focal and segmental glomerulosclerosis(FSGS) were presented with nephrotic range proteinuria, while 45% mesangio-capillary glomerulonephritis, 46% of IgA nephropathy and 32% of mesangial proliferative glomerulonephritis were presented with nephrotic range proteinuria [19].
In this present study out of 100 study cases; 54(54%) cases were presented with renal failure and rest 46(46.0%) cases were presented with normal renal function. Among the proliferative glomerulonephritis group; 78.3% of mesangio-capillary glomerulonephritis, 50% of IgA nephropathy, 52.9% of mesangial proliferative glomerulonephritis and 100% of chronic sclerosing glomerulonephritis cases were presented with renal failure. On the other hand, 100% of minimal change disease (MCD), 80% of membranous glomerulonephritis and 47.4% of focal and segmental glomerulosclerosis (FSGS) cases had normal renal function. Renal function was also normal in 50% of IgA nephropathy, 47.1% of mesangial proliferative glomerulonephritis and 21.7% of mesangio-capillary glomerulonephritis cases. While, 52.6% of focal and segmental glomerulosclerosis (FSGS) and 20% of membranous glomerulonephritis patients were presented with impaired renal function. In this context one previous study reported that, 37% of mesangio-capillary glomerulonephritis, 30% of mesangial proliferative glomerulonephritis, 21% of IgA nephropathy, 30% of focal and segmental glomerulosclerosis (FSGS), 17% of membranous glomerulonephritis patients were presented with renal failure [19]. In this series Bernieh et al. found 36.5% of primary glomerulonephritis presented with renal failure which was an agreement to this present study [6]. It was reported that most proliferative types of primary glomerulonephritis were presented with renal failure [6,19].
In this present study it was found that most of the subgroup of proliferative glomerulonephritis presented with hypertension, 2+ protein in urine, more than 10 RBC/HPF and granular cast in urine, in comparison to non-proliferative subgroups of glomerulonephritis. In this current study it was also found that non-nephrotic range proteinuria was mostly frequent in different subgroups of proliferative glomerulonephritis such as IgA nephropathy (87%), chronic sclerosing glomerulonephritis (67%), mesangial proliferative glomerulonephritis (51%) and mesangio-capillary glomerulonephritis (52% ) in comparison to non-proliferative subgroups that was statistically significant (p<0.01). In this present study patients in different subgroups of proliferative glomerulonephritis presented with raised (>120 µmol/L) serum creatinine level in comparison to non-proliferative group (serum creatinine level <120µmol/L). It was observed that, 100% of chronic sclerosing glomerulonephritis, 80% of mesangiocapillary glomerulonephritis, 50% of IgA nephropathy and 53% of masangial proliferative glomerulonephritis patients were significantly presented with renal failure (p<0.01). Therefore this study demonstrated that, urinary sediment examination along with other clinical and biochemical parameters may help to assume prolierative type of glomerulonephritis rather than non-proleferative type of glomerulonephritis. Although, accurate diagnosis and to plan for treatment strategy and to know the progression of disease, renal biopsy is mandatory and gold standard.
Conclusion
This study concluded that, very active urinary sediments like- RBC, WBC, granular cast, RBC cast and degree of proteinuria along with hypertension and impaired renal function may help us to predict proliferative type of glomerulonephritis from non-proliferative variety of glomerulonephritis. Urinary sediments analysis along with other clinical and biochemical parameters can help us to distinguish proliferative types of glomerulonephritis, but specific variety of proliferative glomerulonephritis is difficult to confirm by urinalysis. Therefore, urinalysis has a great value where biopsy is not feasible because of financial constraints and unavailability of facilities for renal biopsy. In these circumstances, at least we can assume whether the type of proliferative or non-proliferative glomerulonephritis from urinary sediments examination findings along with other clinical and biochemical parameters.
Limitations
It was a single center study and a relatively small sample size.
Recommendation
A multicenter large population based study should conduct to confirm the results of this current study.
Conflict of interest
The authors declared that they have no conflict of interest regarding this publication.
References
- Vinen CS, Oliveira DB. Acute glomerulonephritis. Postgraduate medical journal79(2003):206-213.
- Braden GL, Mulhern JG, O'Shea MH, et al. Changing incidence of glomerular diseases in adults. American journal of kidney diseases 35 (2000):878-883.
- Walbaum D, Kluth D. Presentation of renal disease. Medicine7 (2007):353-358.
- Macanovic M, Mathieson P. Primary glomerular disease. Medicine 35 (2007):489-496.
- Christian MT, Watson AR. The investigation of proteinuria. Current Paediatrics 14 (2004):547-555.
- Bernieh B, Sirwal IA, Abbadi MA, et al. The spectrum of glomerulonephritis in adults in MadinahMunawarah region. Saudi Journal of Kidney Diseases and Transplantation 11 (2000):455.
- Alfonzo JP, Landells JW, Daniel S. Glomerulonephritis: A preliminary clinico-pathological report on 29 patients. Ethiopian Medical Journal 20 (1982):27-32.
- Madaio MP, Harrington JT. The diagnosis of glomerular diseases: acute glomerulonephritis and the nephrotic syndrome. Archives of internal medicine 161 (2001):25-34.
- Al Arrayed A, George SM, Malik AK, et al. Renal biopsy findings in the kingdom of Bahrain: A 13-year retrospective study. Saudi Journal of Kidney Diseases and Transplantation 15 (2004):503.
- Bhagyalakshmi A, Sirisha O, Uma P, et al. Role of urine sediment cytology in the diagnosis of renal disorders in comparison with biochemical and histopathological findings. International Journal of Research in Medical Sciences2(2014): 560-568.
- Mason PD, Pusey CD. Fortnightly Review: Glomerulonephritis: diagnosis and treatment 309(1994): 1557-1563.
- Walbaum D, Kluth D. Clinical assessment of renal disease. Medicine 35 (2007):353-358.
- Priscilla KS, Kenneth F. The investigation of hematuria. SeminNephrol 25 (2005):127-135.
- Nguyen GK. Urine cytology in renal glomerular disease and value of G1 cell in the diagnosis of glomerular bleeding. Diagnostic cytopathology29(2003):67-73.
- Ahmed ZU, Fakir AH. Clinicopathological analysis of glomerulonephritis. Bangladesh Armed Force Medical Journal17 (1993):51-54.
- Alam MR, Khanam A, Alam KS, et al. Prevalence of co-morbidity in hemodialysis patients. Bangladesh Renal Journa 23 (2004):56.
- Balakrishnan N, John GT, Korula A, et al. Spectrum of biopsy proven renal disease and changing trends at a tropical tertiary care centre 1990-2001. Indian Journal of Nephrology 13 (2003):29.
- Vinen CS, Oliveira DB. Acute glomerulonephritis. Postgraduate medical journal 79 (2003):206-213.
- Khanam A, Alam MR, Islam S, et al. Histological pattern of glomerulonephritis in a teaching hospital. Bangladesh23 (2004): 14-18.
- Huraib S. The spectrum of renal disease found by kidney biopsies at King Khalid University Hospital. Saudi Kidney Dis Transpl Bull 1 (1990):15-19.
- Akhtar M, Qunibi W, Taher S, et al. Spectrum of renal disease in Saudi Arabia. Annals of Saudi Medicine. 10 (1990):37-44.
- Yahya TM, Pingle A, Boobes Y, et al. Analysis of 490 kidney biopsies: data from the United Arab Emirates Renal Diseases Registry. Journal of nephrology 11 (1998):148-150.
- Sinniah R. Renal disease in Singapore with particular reference to glomerulonephritis in adults. Singapore medical journal 21 (1980):583-591.
- Naini AE, Harandi AA, Ossareh S, et al. Prevalence and clinical findings of biopsy-proven glomerulonephritidis in Iran. Saudi journal of kidney diseases and transplantation 18 (2007):556.
- Colattur SN, Korbet SM. Long-term outcome of adult onset idiopathic minimal change disease. Saudi Journal of Kidney Diseases and Transplantation 11 (2000):334.
