Analysis of Prescribing Patterns in Paediatric Respiratory Tract Infections with the Focus on Antimicrobial Use, Adverse Effects and Cost Of Drug Therapy
Article Information
Mrinali Thakur1, Rima Shah2*, Darshan Dave3, J G Buch4
1Intern doctor, GMERS Medical College, Gandhinagar, Gujarat, India
2Assistant Professor, Department of Pharmacology, GMERS Medical College, Gandhinagar, India
3 Professor and Head, Department of Pharmacology, GMERS Medical College, Gandhinagar, India
4Dean & Professor Pharmacology, Dharmsinh Desai Medical Institute and Hospital, Nadiad, Gujarat, India
*Corresponding author: Rima shah, Department of Pharmacology, GMERS Medical College, Gandhinagar, India
Received: 19 February 2020; Accepted: 03 March 2020; Published: 06 March 2020
Citation: Mrinali Thakur, Rima Shah, Darshan Dave, J G Buch. Analysis of Prescribing Patterns in Paediatric Respiratory Tract Infections with the Focus on Antimicrobial Use, Adverse Effects and Cost Of Drug Therapy. Journal of Pharmacy and Pharmacology Research 4 (2020): 001-014.
View / Download Pdf Share at FacebookAbstract
Aim: To analyze the pharmacological management of respiratory tract infections (RTIs) in paediatric patients.
Methodology: A cross-sectional observational study involving 74 patients of paediatric RTI was carried out in a tertiary care teaching hospital. Patients’ demographic and disease related details, drug history, adverse drug reactions, cost of therapy were noted in structured case record form and analysed. Appropriateness of treatment was analysed by comparing with the guidelines set by the WHO and the Indian Academy of Pediatrics (IAP).
Results: Out of total 74 patients, 54.05% were in the age group of <1 year and 67.57% were male. The most common diagnosis was pneumonia (48.65%). Average number of drug per patient was 7.25±1.57 (range 3 to 16). Most common drug groups prescribed were antibacterials(100%), analgesic/antipyretics(95.94%) and respiratory drugs(86.49%). Among the antibacterials, amoxicillin+clavulanic acid(90.54%) and ceftriaxone(77.77%) were frequently prescribed. Among respiratory medicines, antihistamines and salbutamol were prescribed to 85.13% and 55.40% of patients respectively. 56.81% drugs prescribed by their generic name and 75% of drugs were prescribed from WHO-EML. Appropriate/rational drug therapy was given to only 13.51% of the patients while drug therapy of rest 35.14% and 51.35% patients was found to be semi-rational and irrational respectively as per the WHO and IAP guidelines. 16.22% patients developed an ADR which was due to antimicrobial or analgesic/antipyretics. Average total drug cost per indoor patient was 314.69 Rs and total antimicrobial cost was calculated as Rs. 286.17.
Conclusion: An overuse of antibacterials and respiratory medicines was seen in the study. Emphasis on proper diagnosis and treatment, education and availability of locally effective guidelines may
Keywords
Paediatric respiratory tract infection; Antimicrobial use; Appropriateness of prescribing; Cost analysis of drug therapy; Adverse reactions in paediatrics
Paediatric respiratory tract infection articles, Antimicrobial use articles, Appropriateness of prescribing articles, Cost analysis of drug therapy articles, Adverse reactions in paediatrics articles
Article Details
Introduction
Respiratory tract infections are the major cause of morbidity and mortality in the paediatric age group in developed as well as developing countries [1]. In India they are responsible for 20% of the under-five mortality, as compared to only 3% in the developed countries [2]. India has the highest child birth rate as well as child death rate [2]. In the year 2010, the under 5 deaths in the world was about 1.7 million, among which 23% death rate belonged to India [2]. Among this death rate, pneumonia constitutes about 24% of the total under 5 death rate in our country [2,3]. One of the ‘Sustainable Development Goals’ launched by United Nations in 2016 is to reduce under-five mortality rate to at least 25/1000 live births by the year 2030. It also targets towards decreasing preventable deaths and epidemics of communicable diseases by year 2030 [3]. Hence, it is important to try and treat maximum children to decrease their chances of mortality.
Respiratory tract infections include wide range of pathological conditions like bronchiolitis, pneumonia, bronchitis, laryngotracheobronchitis, acute sinusitis, otitis media, rhinitis etc. They can be of viral (adenovirus, rotavirus, rhinovirus etc) or bacterial (H. influenzae, Streptococci, pneumococci, Moraxella catarrhali etc) origin.[4] Treatment modalities include use of antimicrobials (penicillins, erythromycin, cephalosporins etc), respiratory medicines (salbutamol, ipratropium bromide), cough and cold medicines (nasal decongestants, anti-histaminics etc) or symptomatic management [4,5].
Emergence of newer pathogenic organisms, re-emergence of disease previously controlled, widespread antibiotic resistance and suboptimal immunization coverage even after many innovative efforts are the major factors responsible for high incidence of respiratory tract infections [3]. It therefore becomes the top most priority to prevent and treat these infections. Although the practice guidelines are available for all respiratory infections, the extent of their implementation and effectiveness has not yet been analysed [6]. Also most of the guidelines are based on the etiology, but in most developing countries, including India, the therapy is usually empirical [7]. This can lead to consequences like irrational use of antimicrobials and drug resistance [8].
Respiratory tract infection can not only affect an individual specifically but also affects the society by increase chances of spreading the infection through infected airborne droplets. Given the high incidence of respiratory tract infections in the paediatric age group and lack of studies in this field, present study has been planned to analyse the prescribing pattern of the drugs in paediatric respiratory tract infection patients, adverse effects and the cost of drug therapy.
Methodology
This was a prospective, observational, single centre study, undertaken on paediatric inpatients of respiratory infections at Civil Hospital, Gandhinagar, a tertiary care teaching hospital in western India. The research protocol was presented to the Institutional Ethics Committee (IEC) and approval was taken before commencement of the study. Parents/guardians of the children were explained clearly about the nature and purpose of the study in the language they understood. Written informed consent was obtained from the parent/guardian before enrolling the patient for the study. Permission from Medical Superintendent and the Head of the paediatric department was obtained before conducting the study.
Sample size: Considering, the prevalence of respiratory tract infections in paediatric patients (5%) in India [2], the sample size calculated is 73 using following formula:
Where, 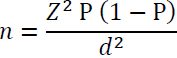
Z = Z statistics for level of confidence (95%) = 1.96
P = Expected Prevalence = 0.05 (= 5%)
d = Precision = 0.05 (= 5%)
Patient selection criteria
Children aged between 1 months to 12 years and of either gender and diagnosed to be suffering from acute respiratory tract infection and admitted to paediatric department (both ward and ICU) of the civil hospital, Gandhinagar during the study period of March to August 2016 were enrolled for the study. Patients diagnosed with Hospital acquired respiratory infection and those patients who take discharge against medical advice (DAMA) were excluded from the analysis.
Study procedure
Patients admitted to paediatric ward and intensive care units with respiratory infections were screened and those meeting inclusion and exclusion criteria were enrolled for the study. All the patients will be visited daily and their treatment was recorded till the patient was discharged from the hospital. All necessary information like demographic data (age, gender, socioeconomic status etc) clinical data (history of illness, symptoms and signs, duration of stay) and drug treatment (dosage regimen) was collected by reviewing the hospital case file and by interview with parents/children. All the information gathered was recorded in the structured case record form. A small pilot study on 10 patients was carried out first to test the predesigned case record form (CRF). The case record form was then be modified accordingly to fillup all patients’ data.
For analysis of ADRs: All the adverse drug reactions spontaneously reported or identified by the investigator was monitored and reported. Causality assessment of the ADRs was done using the WHO-Uppsala Monitoring Center (UMC) scale [9] and Naranjo’s algorithm [10]. The severity and preventability of the ADRs was assessed by using the Hartwig and Siegle scale [11] and the modified Schumock and Thornton criteria [12] respectively.
For analysis cost of drug therapy: Cost of medication was obtained from the hospital (if supplied by the hospital) or private pharmacies (if unavailable in the hospital pharmacy). The cost of medicines was calculated for complete hospital stay.
For analysis of appropriateness of therapy: Drug treatment prescribed was compared with the WHO treatment guidelines [13, 14] and Indian Association of Paediatrics (IAP) guidelines [15]. The drug treatment was considered as rational if it is followed as per guidelines; semirational if rational alternatives were used and irrational if not as per the standard treatment guidelines.
Proposed format for data analysis
All the gathered data was analysed for (i) Demographic parameter, age and gender wise distribution of patients, (ii) prevalence of different diseases causing respiratory infections, (iii)clinical presentation: symptoms and signs (iv) duration of hospital stay (v) drug treatment given with its route, dose, frequency and duration of treatment and prescribed by generic name or brand name, included in EML or not (vi) analysis of antimicrobials used, type of use: empirical / definitive, its source (whether from hospital supply or from outside pharmacy), (vii) appropriateness of drug therapy (viii) analysis based on WHO-core prescribing indicators [16] (ix) adverse drug reactions and (x) calculation of cost of drug therapy
Statistical analysis
The data thus collected was subjected to statistical analysis using Microsoft excel 2010. Data were expressed in actual frequencies, percentage, mean, standard deviation as appropriate.
Results
A total of 74 patients of respiratory tract infection admitted in paediatric indoor unit and meeting inclusion-exclusion criteria were enrolled over the period of 6 months (March-August 2014). The mean age of the patients was 2.36 ± 2.12 years with a range of 1 month to 11 years. Most patients (54.05 %) were less than 1 year of age. Out of these 74 patients, 50 (67.57 %) were males and 24 (32.43%) were females. A total of 66 (89.19%) patients were fully immunized as per the National Immunization Schedule. Baseline parameters and demographic details of the patients had been shown in table 1.
|
Parameter |
No. of patients (n=74, %) |
|
Age in years |
|
|
0-1 |
40 (54.05) |
|
1-2 |
9 (12.17) |
|
2-5 |
8 (10.81) |
|
5-10 |
14 (18.92) |
|
>10 |
3 (4.05) |
|
Gender |
|
|
Male |
50 (67.57) |
|
Female |
24 (32.43) |
|
Body weight in Kg |
|
|
1 – 5 |
20 (27.02) |
|
5.1 – 10 |
25 (33.78) |
|
10.1 - 15 |
13 (17.57) |
|
15.1 – 20 |
9 (12.16) |
|
20.1 – 25 |
4 (5.41) |
|
>25 |
3 (4.05) |
|
Immunization status |
|
|
Complete |
66 (89.19) |
|
Partial |
3 (4.05) |
|
Non-immunized |
2 (2.71) |
|
Unknown |
3 (4.05) |
Table 1: Demographic parameters of the study population: (n=74)
Majority (56, 75.68) patients were diagnosed as suffering from lower respiratory tract infection while rest 24.32 % had upper respiratory tract infection like pharyngitis and tonsillitis. Among LRTI, pneumonia was most common cause for admission to hospital followed by bronchiolitis and WALRTI as shown in table 2.
Common symptomatology of patients with respiratory diseases is shown in table 2. Most common presenting symptom was cough and cold in 94.55% patients, followed by fever and breathlessness in 90.54% and 24.32% of patients respectively. Thorough history was followed by different laboratory investigations for identification of the cause of respiratory infections. Complete blood count was carried out in 69 (93.24%) of patients followed by x-ray chest in 53 (71.62%) patients. Duration of hospital stay ranged from 3 to 11 days with an average of an average of 4.3 ± 2.1 days. Majority had duration of hospital stay upto 3-7 days (58, 78.38%).
|
Diagnosis |
No. of patients (%) |
|
URTI (pharangitis, tonsilitis) |
18 (24.32) |
|
LRTI |
56 (75.68) |
|
Pneumonia |
36 (48.65) |
|
Bronchiolitis + bronchitis |
10 (13.51) |
|
Bronchial asthma |
3 (4.05) |
|
WALRTI |
7 (9.46) |
|
Symptoms |
|
|
Fever |
67 (90.54) |
|
Cough and cold |
70 (94.55) |
|
Breathlessness |
18 (24.32) |
|
Duration of stay (in days) |
|
|
1-3 |
4 (5.41) |
|
3-7 |
54 (72.97) |
|
> 7 |
16 (21.62) |
Table 2: Disease related parameters in study patients: (n=74)
Analysis of drug use
The average number of drugs prescribed per patient was 7.25 ± 1.57 (range 3 to 16). Out of these 74 patients, 14 (18.92%) were prescribed less than 5 drugs while polypharmacy (upto 5-10 drugs) and high polypharmacy (>10 drugs) was prevalent in 53 (71.62%) and 7 (9.46%) of patients respectively.
Seven different conditions were found to be prevalent in total 74 patients. Different 36 drugs were prescribed as 88 formulations in 74 cases. Out of the 88 formulations, 38 (43.18%) and 50 (56.81%) were prescribed by brand and generic names, respectively.
Most common drug group prescribed was antimicrobial agents in all patients followed by analgesic antipyretics (95.94%) and respiratory medicines (86.49%) as shown in table 3 and 4. Number of antimicrobial agents prescribed was ranged from 1 - 4. Single antibiotic was prescribed in 48.65% of patients only. Most common antibiotic used was amoxicillin plus clavulanic acid in 90.54 % of patients followed by cephalosporins in 48.65% and aminoglycosides in 24.32% of patients. Duration of antibiotic use was 1-10 days. Analgesic/antipyretic was required in 71 (95.95%) patients, of which 68 received paracetamol and 3 patients received paracetamol plus ibuprofen. Duration of use of analgesic/antipyretics ranged from 1-10 days (Table 4).
|
Drug |
No. of patients (%) |
|
Antibiotics |
74 (100) |
|
Number of antibiotics prescribed |
|
|
1 |
36 (48.64) |
|
2 |
20 (27.02) |
|
3 |
16 (21.62) |
|
4 |
2 (2.70) |
|
Class of antibiotics used |
|
|
Amoxicillin + clavulanic acid |
67 (90.54) |
|
Amoxicillin |
4 (5.40) |
|
Ampicillin |
6 (8.10) |
|
Aminoglycosides (amikacin) |
18 (24.32) |
|
Cephalosporins |
36 (48.64) |
|
Ceftriaxone Cefixime Cefotaxime |
28 (77.77) 3 (8.33) 5 (13.88) |
|
Azithromycin |
2 (2.70) |
|
Antihelmintic drugs (Albendazole) |
8 (10.81) |
|
Diethylcarbamazine (DEC) |
2 (2.70) |
|
Duration of antibiotic use |
1-10 days |
Table 3: Analysis of antimicrobial drugs used in study patients (n=74)
|
Supporting medicines |
No. of patients (%) |
|
Oxygen |
26 (35.13) |
|
IV fluids |
28 (37.83) |
|
Analgesics/antipyretics |
71 (95.94) |
|
Paracetamol Paracetamol + Ibuprofen Duration of use |
68 (95.77) 3 (4.05) 1-10 days |
|
Respiratory drugs |
64 (86.49) |
|
Salbutamol Antihistaminic drugs Cetirizine Chlorphenaramine maleate Phenaramine maleate Saline nasal drops Dextromethorphan Doxophyllin Cough syrup |
41 (55.40) 63 (85.13) 19 (30.15) 41 (65.07) 3 (4.76) 4 (5.40) 12 (16.21) 2 (2.70) 2 (2.70) |
|
GIT drugs |
44 (59.45) |
|
H2 blockers PPI |
24 (54.54) 2 (4.54) |
|
Ondensetron Domperidone |
34 (81.81) 2 (4.54) |
|
ORS |
11 (14.86) |
|
Steroids |
11 (14.86) |
|
Dexamethasone Hydrocortisone Prednisolone Budesonide |
2 (18.18) 4 (36.36) 4 (36.36) 5 (45.45) |
PPI - proton pump inhibitors, ORS – oral rehydration salt solution
Table 4: Analysis of supportive medicines used in the study patients: (n=74)
World health organization core indicators
As per WHO core indicators, it was observed that polypharmacy was widely practiced. Antibacterials were prescribed in all patients. Similarly, almost all patients were prescribed injections. More than half the drugs were prescribed by their generic names. A majority of the drugs prescribed were from the National Essential Medicines List (EML) 2011 (86.11%) and the WHO EML 2010 (75%). An analysis of drugs prescribed is given in Table 5.
|
WHO core indicators |
No. of patients (%) |
|
Number of drugs prescribed per encounter (mean±SD) |
7.25 ± 1.57 |
|
Number of drugs prescribed by generic name (%) |
50 out of 88 formulations (56.81) |
|
Number of encounters resulting in the prescription of an antibacterial (%) |
74(100) |
|
Number of encounters resulting in the prescription of an injection (%) |
74 (100) |
|
Number of drugs prescribed from the National Essential Medicines List 2011 (%) |
31 out of 36 drugs (86.11) |
|
Number of drugs prescribed from WHO EML 2010 (%) |
27 out of 36 drugs (75) |
(WHO=World health organization, EML=Essential medicines list)
Table 5: WHO core prescribing indicators for pediatric inpatients (n=74)
Evaluation of appropriateness
While comparing with the WHO respiratory disease management guidelines and IAP guidelines, treatment given to only 10 (13.51%) of the patients could be considered rational while rest 26(35.14%) could be considered as semi-rational and 38 were irrational (51.35%).
Adverse drug reactions
Out of these 74 patients, 12(16.22%) developed an ADR. Out of these 8 patients developed diarrhoea, 1 developed severe gastritis and vomiting and 3 patients developed maculopapular rash with itching. They all fell in category of “possible” according to WHO causality assessment criteria and Naranjo’s probability scale. All can be attributed to use of antimicrobials and/or analgesic-antipyretics prescribed. All the ADRs could be considered as moderate in severity and none of the ADR was classified as preventable ADR. Diarrhoea was treated with ORS, for gastritis intravenous pantoprazole was used and rash was treated with syrup phenaramine maleate. For treatment of vomiting tablet or syrup domperidone or ondensatrone was prescribed. All patients recovered from the ADRs with the given treatment.
Cost of treatment
Average drug cost per indoor patient amounted to a total of Rs. 314.69. Out of this total drug cost, total antimicrobial cost was calculated as Rs. 286.17. Total drug cost for all 74 patients is calculated as 23287.06 Rs.
Discussion
The present study was done with an intention of analyzing the pharmacological management of respiratory tract infections in hospitalized children. The majority of enrolled patients were less than one year of age, which may be due to increased susceptibility to infections during the weaning period. The most frequent presenting complaints were cough, fever, and breathlessness, signifying the serious condition of the patient, requiring hospitalization. The common diagnoses were pneumonia and bronchiolitis, which have been noticed in Brazil, where nearly 30% of the admissions were due to pneumonia. [17] No distinction was made between bacterial and viral etiologies in the diagnoses based on culture and sensitivity testing but all the patients was evaluated using complete blood count. If total white blood cell count were higher than normal, it was considered as bacterial origin and treated accordingly. This is important as viral pneumonia as well as bronchiolitis usually do not require antibacterial treatment.
A great deal of polypharmacy was evident as the average number of drugs prescribed per patient was 7.25 (range 3-16). Antibacterials were observed to be the most frequently prescribed drugs, as was also observed in a study conducted in western Nepal, [18] followed by analgesic/antipyretics and respiratory medicines. Amoxicillin with clavulanic acid was prescribed in 90.54% of patients followed by cephalosporins in 48.64% of patients. This finding is comparable to other studies reported from Kathmandu [19] and Palestine [4], where either penicillin or cephalosporin were the most frequently prescribed antibacterials for pediatric inpatients. In these studies, older penicillin was used more frequently (crystalline penicillin and ampicillin), which can be attributed in part to the variation in the local resistance pattern. Also these studies were conducted around ten years earlier. Prescription of two and more antibacterials found in almost half of the patients. Clearly the hassle of identifying the organism was avoided and several antibacterials were prescribed concomitantly to cover all possible organisms leading to greater chances of adverse drug reactions, drug interactions, development of resistance and increased cost of therapy [20].
All the patients in the study were prescribed with supportive medicine like oxygen, analgesic antipyretics. Supportive medications that target symptoms of viral URIs are used widely in patients with rhinosinusitis, particularly in the early stage of infection. There is a lack of evidence supporting their use RTIs, but they may provide temporary relief in certain patients [7,8]. Analgesics can be used to treat fever and pain from sinus pressure or malaise. Oral decongestants relieve congested nasal passages but should be avoided in children younger than 2 years of age and patients with ischemic heart disease or uncontrolled hypertension. Intranasal decongestants can be used for severe congestion in most patients 6 years of age or older, but use should be limited to 3 days or less to avoid rebound nasal congestion [21]. Antihistamines should be avoided because they thicken mucus and impair its clearance, but they may be useful in patients with predisposing allergic rhinitis or chronic sinusitis [21]. In this study, 85.13% of patients were prescribed antihistaminic drugs, which suggests over use of these drugs.
Salbutamol was prescribed to 55.40% patients in this study. Use of bronchodilators in pneumonia and bronchiolitis is not well established [22]. These drugs were used to provide symptomatic relief, particularly in those cases where a significant amount of wheeze is seen. It was also observed that almost 50% of the respiratory medicines were given to children below one year of age, which included cough and cold combinations. A Cochrane review of the efficacy of these combinations concluded that they were no more effective than placebo in children [21]. The US FDA has banned the use of these medicines in children less than two years of age [23]. The threat of overdose, combined with their lack of proven benefit in children does not support their use for relieving respiratory symptoms. Symptomatic and supportive therapy in the form of antipyretics and intravenous fluids has been used appropriately in these patients. Steroids have been prescribed only in cases of acute laryngo-tracheo-bronchitis (reduces laryngeal edema) and asthma which could be considered as appropriate but its use in lobar pneumonia was ineffective and inappropriate [24].
In present study, 16.22% patients had developed one or other ADR which is higher than other reports from the India [25]. It should be mentioned that the ADRs seen in our study were caused by antibacterials, as was also seen in Spain, [26] Brazil, [27] and Italy, [28] thus substantiating the widely acknowledged fact that irrational use of antibacterials led to adverse drug reactions. Another fact noted was that around half of all ADRs were non-preventable in nature while in other half ADRs either the causal drugs were not indicated in the patients or preventive measures were not taken. All ADRs were reported by the investigator and none of them reported by the patient or treating physician (spontaneous reporting). Lack of suspicion that a complaint could be due to an ADR might be the reason for the low reporting of ADRs by treating physicians. Even the patients themselves might not understand the correlation of a particular complaint (ADR) and the drug, and hence, not bring the reaction to the notice of the pediatrician.
Average drug cost per indoor patient amounted to a total of Rs. 314.69 in this study. Out of this total drug cost, total antimicrobial cost was calculated as Rs. 286.17. Total drug cost for all 74 patients is calculated as 23287.06 Rs. Although significant, it was still less compared to other countries. In Germany, the total cost per hospitalized pediatric patient with community-acquired pneumonia was found to be €2579 (Rs. 153,778),[29] while in Pakistan the average cost per episode ranged from $22 to $142 depending on the severity of the disease [30]. A difference in the healthcare policies between countries (reimbursements, higher monetary value for productivity) and nature of the study (our study is prospective, while most of other studies were retrospective) may account for this discrepancy. Considering the high disease burden, the UNICEF and WHO have jointly developed a Global Action Plan for the prevention and control of Pneumonia (GAPP) [31], in 2009, which has focused on developing countries and allocates monetary funds for its control.
This study has identified the common diseases causing respiratory tract infections in paediatric patients and effectively highlighted the prescribing patterns and adverse drug reactions. Present study was conducted in single centre and for shorter duration of time which was the limitation of the study. Also detailed analysis of direct cost, indirect cost and disease burden on the hospital and community could not be calculated. Overall, such drug utilization Studies can help in identifying the prevailing scenario of drug prescribing patterns, different measures to facilitates the rational use of medicines and also ensures the prudent use of the available resources. As seen in our study, antibacterials and respiratory medicines are often used indiscriminately. Education of the prescribers as well as the caregivers is imperative. Even as the heavy workload in Indian hospitals may be cited as a reason for the inability of doctors to communicate effectively with the parents, an effort must always be made in this regard, particularly with the help of paramedical workers.
This study has found the need for a standard treatment guideline for our own hospital, taking into account the local sensitivity pattern of the organisms. Although culture and antibiotic susceptibility were not performed in our study to confirm the rationality of the antibacterial used, the present study serves to highlight the current treatment practice of these infections in our hospital and pave the way for further interventions that can help implement the rational use of medicines.
Conclusion
Pneumonia is the most common cause of admission among respiratory tract infections in paediatric patients. Identification of etiology of the infection is very important in management of patients with respiratory tract infections. Viral illnesses are self-limiting and require only supportive therapy. Misuse of antimicrobials is an alarming problem requiring attention of all health care professionals. Appropriate selection of an antimicrobial agent and its dosage regimen can reduce morbidity and mortality. There is a need for developing standard treatment guidelines for the hospital considering local sensitivity pattern. Education of the prescribers and care givers about rational therapeutics can help in reducing inappropriate antimicrobial use, thereby reducing preventable ADRs and reduce cost of treatment.
Acknowledgement
We sincerely thank all staff members of paediatric inpatient department for giving cooperation and for their help in conducting patients’ interview.
References
- Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 86 (2008): 408-16.
- World Health Organization. World Health Statistics 2010. France: WHO;2010.
- United Nations. Sustainable development Goals 17 goals to transform our world. Available from: http://www.un.org/sustainabledevelopment/sustainable-development-goals/ Last accessed on 21st January 2016
- Sawalha A, Al-Bishtawi G, Al-Khaayyat L, Sweileh W, Al-Ramahi R,J aradat N. Pattern of parenteral antimicrobial prescription among pediatric patients in Al-Watani Government hospital in Palestine. An-Najah Univ J Res (N.Sc.) 20 (2006): 191-206.
- Food and Drug Administration. FDA releases recommendations regarding use of over-the-counter cough and cold products. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116839.html. [Last updated on 2009 Jun 18; Last accessed on 2009 Aug 16].
- Worrall G, Chaulk P. Hope or experience? Clinical practice guidelines in family practice. J FamPract 42 (1996): 353-356.
- Hart CA, Kariuki S. Antimicrobial resistance in developing countries. BMJ 317 (1998): 647-50.
- Ashraf H, Handa S, Khan NA. Prescribing pattern of drugs in outpatient department of child care centre in Moradabad city. Int J Pharm Sci Rev Res 3 (2010): 1-5.
- World Health Organization. WHO-UMC system for standardized case causality assessment. Available from: http://www.who-umc.org/graphics/4409.pdf. [Last accessed on 2009 Dec 12].
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30 (1981): 239-45.
- Hartwig S, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 49 (1992): 2229-32.
- Lau PM, Stewart K, Dooley MJ. Comment: Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother 37 (2003): 303-5.
- Cough and difficult breathing. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. Geneva: WHO? 2005.
- Technical updates of the guidelines on the Integrated Management of Childhood Illness (IMCI): evidence and recommendations for further adaptations. Geneva? WHO? 2005.
- Yadav KK, Awasthi S. The current status of community-acquired pneumonia management and prevention in children under 5 years of age in India: a review. Ther Adv Infectious Dis 3 (2016): 83–97.
- Richard Ofori-Asenso. A closer look at the World Health Organization's prescribing indicators. J Pharmacol Pharmacother 7 (2016): 51–54.
- Santos DB, Clavenna A, Bonati M, Coelho HL. Off label and unlicensed drug utilization in Fortaleza, Brazil. Eur J Clin Pharmacol 64 (2008): 1111-8.
- Shankar PR, Upadhyay DK, Subish P, Dubey AK, Mishra P. Prescribing patterns among pediatric inpatients in a teaching hospital in western Nepal. Singapore Med J 47 (2006): 261.
- Palikhe N. Prescribing pattern of antibiotics in pediatric hospital of Kathmandu valley. J Nepal Health Res Counc 2 (2004): 31-6.
- Vashishtha VM. Growing antibiotic resistance and need for new antibiotics. Indian Pediatr 47 (2010): 505-6.
- Smith SM, Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev 2008: CD001831.
- Gadomski AM, Brower M. Bronchodilators for bronchiolitis (Review). Cochrane Database Syst Rev 2010: CD001266.
- Food and Drug Administration. FDA releases recommendations regarding use of over-the-counter cough and cold products. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116839.html. [Last accessed on 2016 Aug 16].
- Salluh J, Povoa P, Soares M, Castro-Faria-Neto HC, Bozza FA, Bozza PT. The role of corticosteroids in severe community-acquired pneumonia: A systematic review. Crit Care 12 (2008): R76.
- Iyer GS, Patel PP, Panchal JR, Dikshit RK. An analysis of the pharmacological management of respiratory tract infections in pediatric in-patients at a tertiary care teaching hospital. Int J Med Public Health 3 (2013): 140-5.
- Martinez-Mir I, Garcia-Lopez M, Palop V, Ferrer JM, Rubio E, Morales-Olivas FJ. A prospective study of adverse drug reactions in hospitalized patients. Br J Clin Pharmacol 47 (1999): 681-8.
- Dos Santos DB, Coelho HL. Adverse drug reactions in hospitalized children in Fortaleza, Brazil. Pharmacoepidemiol Drug Saf 15 (2006): 635-40.
- Clavenna A, Bonati M. Adverse drug reactions in childhood: A prospective studies and review of safety alerts. Arch Dis Child 94 (2009): 724-8.
- Ehlken B, Ihorst G, Lippert B, Rohwedder A, Peterson G, Schumacher M et al. Economic impact of community-acquired and nosocomial lower respiratory tract infections in young children in Germany. Eur J Pediatr 164 (2005): 607-15.
- Hussain H, Waters H, Khan AJ, Omer SB, Halsey NA. Economic analysis of childhood pneumonia in northern Pakistan. Health Policy Plan 23 (2008): 438-42.
- United Nation’s Children Emergency Fund, World Health Organization. Technical Consensus Report. Global action plan for the prevention and control of pneumonia. Report of an informal consultation. UNICEF, 2009.
