A Pediatric Case of IgA Nephropathy Associated with Familial Mediterranean Fever
Article Information
Marwa Chbihi1*, Cécile Dumaine2, Georges Deschênes1, Anne Couderc1, Renato C Monteiro3, Julien Hogan1, Alexandra Cambier1*
1Pediatric Nephrology Department, Robert Debré Hospital, Paris, France
2Pediatric Department, Robert Debré Hospital, Paris, France
3Immunoreceptors and renal immunopathology, Paris Diderot University, Paris, France
*Corresponding Authors: Dr. Marwa Chbihi, Pediatric Nephrology Department, Robert Debré Hospital, Paris, France
Dr. Alexandra Cambier, Pediatric Nephrology Department, Robert Debré Hospital, Paris, France
Received: 23 January 2020; Accepted: 10 February 2020; Published: 05 March 2020
Citation: Marwa Chbihi, Cécile Dumaine, Georges Deschênes, Anne Couderc, Renato C Monteiro, Julien Hogan, Alexandra Cambier. A Pediatric Case of IgA Nephropathy Associated with Familial Mediterranean Fever. Archives of Clinical and Medical Case Reports 4 (2020): 218-225.
View / Download Pdf Share at FacebookAbstract
IgA nephropathy (IgAN) is one of the most common primary glomerulonephritis in children and adolescents. Pharyngitis infections are known to be a trigger of IgA attacks. It has been suggested that IgAN is an immune complex disease with circulating immune complexes galactose-deficient IgA1 (Gd-IgA1), IgG and IgA anti-Gd-IgA1 antibodies, and soluble CD89. Amyloidosis is the most common renal complication of Familial Mediterranean Fever (FMF), the most common form of auto-inflammatory syndromes characterized by flares of fever and polyserositis. Besides amyloidosis, other renal lesions have been exceptionally reported in patients with FMF and other hereditary periodic fevers. We report a child who presented with haematuria during febrile attacks in the context of FMF, and in whom kidney biopsy revealed deposits of predominant mesangial IgA with no evidence of amyloidosis and presence of immune complexes in the serum (Gd-IgA and sCD89-IgA).
Keywords
IgA nephropathy; Familial Mediterranean Fever; Inflammation; Chronic kidney disease; Immune complexes
IgA nephropathy articles, Familial Mediterranean Fever articles, Inflammation articles, Chronic kidney disease articles, Immune complexes articles
Article Details
1. Introdusction
IgA nephropathy (IgAN), described by Jean Berger and Nicole Hinglais in 1968 [1], is one of the most common primary glomerulonephritis in children and adolescents particularly in Western and Asian countries [2]. Children IgA nephropathy is a morbid disease. Thirty percent of patients will reach end stage renal disease within 20 years of follow up. There is a growing body of evidence suggesting that IgAN is an immune complex disease, where circulating immune complexes are deposited exclusively in the glomerular mesangium. Circulating immune complexes are composed of galactose-deficient IgA1 (Gd-IgA1), IgG and IgA anti-Gd-IgA1 antibodies, and soluble CD89 (Fc receptor for IgA) [3]. In recent years, clinical studies have attempted to demonstrate a link between clinical manifestations, renal prognosis, and these three main immunologic factors involved in IgAN physiopathology [4, 5]. An infectious disease is usually preceding the nephropathy, which is supposed to lead to a dysregulated immune response. Familial Mediterranean fever (FMF) is the most common form of auto-inflammatory syndromes, and is characterized by recurrent inflammatory attacks of fever and serositis. The gene responsible for the disease is called Mediterranean fever gene (MEFV) and is localised on the short arm of chromosome 16 and encodes a 781-amino acid protein known as pyrin or marenostrin. Amyloidosis is the most common renal complication ofFMF. Besides amyloidosis, other renal lesions have been exceptionally reported in patients with FMF and other hereditary periodic fevers [5].
In this case-report, we describe a child who presented with haematuria during febrile attacks in the context of FMF, and in whom kidney biopsy revealed deposits of predominant mesangial IgA with no evidence of amyloidosis and presence of immune complexes in the serum (Gd-IgA and sCD89-IgA).
2. Case Presentation
We report the case of a 15-year-old boy born to non-related parents. His mother’s history was remarkable for breast cancer and Hodgkin lymphoma. His grandfather, an aunt, a cousin, and one brother also had features of FMF. Since the age of 11 years old, the patient presented recurrent hyperthermia on a bimonthly basis. Hyperthermia was resistant to antipyretic treatment and lasted 4 to 7 days often associated with mouth ulcers. Other symptoms included extreme fatigue, abdominal pain, anorexia, lymphadenopathies and either tonsillitis or pharyngitis. No skin rash, arthritis or myalgia was reported.
Biological exams performed during a flare showed elevated inflammation markers (CRP 37 mg/l, ESR 19 mm/h) with normal blood count and elevated serum amyloid A plasma level (428 mg/l). Hepatic and renal functions were normal. FMF was then highly suspected and a treatment by colchicine at 1 mg per day was initiated, leading to a major decrease in the number of flares, and to the normalization of SAA plasma level. Genetic testing confirmed a heterozygous mutation M694V in exon 10 of MEFV gene.
Soon after the diagnosis, the patient presented with a macroscopic haematuria during a febrile episode. Physical examination confirmed the high fever (39.3°C) and was otherwise unremarkable except for tonsillitis and small cervical lymphadenopathies. Blood pressure was normal. Renal function was altered with an elevated creatinine (107 µmol/L, estimated eGFR with modified Schwarz formula 97 ml/min). There was no evidence of significant proteinuria (0,21 g/l, uP/creat ratio 0,02g/mmol) and serum albumin was subnormal (38 g/L). However, microalbuminuria was significantly increased (123 mg/l; microalbuminuria/creatininuria ratio 10,8mg/mmol). Other investigations concluded to the lack of complement system activation (C3 1.17 g/l C4 0.30 g/l), a former EBV immunisation, negative streptococcus and viral serologies (Parvovirus B19, HAV, HBV, HCV, HIV). Serum IgA level was normal (2,55 g/l).
A renal biopsy was performed, showing mesangial proliferation and focal and segmental glomerulosclerosis lesions with IgA and C3 mesangial deposits on immunofluorescence supporting the diagnosis of IgA nephropathy. Oxford score was: M 1, E0, S1, T0, C0. Red Congo staining excluded amyloidosis deposits. Galactose-deficient IgA were elevated (4195 ng/ml) and soluble CD89-IgA complexes as well as IgG-IgA were positively detected in the plasma (Table 1).
|
Test |
Result |
|
Serum creatinine (umol/l) |
107 |
|
Haemoglobin (g/dl) |
10 |
|
Albumin (g/l) |
38 |
|
Proteinuria (g/l) |
0.21 |
|
Haematuria (/ml) |
>1,000,000 |
|
Serum Ig A (g/l) |
2.55 |
|
Serum galactose-deficient Ig A (ng/ml) |
4195 |
|
Renal biopsy: Oxford classification |
M (1) E0 S1 T0 C0 |
|
Mutation |
M694V in exon 10 of MEFV gene |
Table 1: Investigations at onset IgA nephropathy.
Enalapril was added to colchicine based on the results of the renal biopsy. Although he presented a relapse of fever with macroscopic haematuria 6 months after neither proteinuria nor microalbuminuria were observed at last follow up (microalbuminuria: 10 mg/l). At last follow-up, after one year of treatment with colchicine and enalapril, he remained with an isolated microscopic haematuria (30,000 erythrocytes/ml).
3. Discussion and Conclusion
We report a rare case of FMF associated with IgA nephropathy and documented by genetics, typical clinical history, renal biopsy findings and the positivity of new circulating biomarkers. FMF is an auto-inflammatory disease characterised by fever and inflammation causing pleuritis, peritonitis, arthritis and erythema with a high prevalence in people of Mediterranean origin [7]. It is an autosomal recessive disease, but there are more and more symptomatic patients with heterozygous mutations. One of FMFs most common complications is amyloidosis [8], which essentially affects the kidneys. Renal amyloidosis is a severe complication of FMF and significantly worsens the prognosis of the patients [9]. However, other renal diseases are poorly documented and have been reported in patients with FMF, such as hemorrhagic glomerulonephritis, chronic glomerulonephritis, Henoch-Schönlein nephritis and polyarteritis nodosa [10, 11].
In the 70s, Eliakim et al. analyzed renal manifestations in 106 patients with FMF, and discovered that 66% had no renal lesions, 12.3% had renal amyloidosis, and 21.7% had renal damages other than amyloidosis described as glomerulonephritis lesions; patients in this last category presented with transient or persistent haematuria in combination with albuminuria, particularly during disease flare[12].. Rigante et al. reported the first pediatric case of FMF associated with IgA nephropathy only based on the biopsy result [13]. Studies have reported the presence of granular patterns of mesangial IgA deposits in two patients with FMF displaying proteinuria, microscopic hematuria, and elevated serum IgA levels during their febrile attacks [14, 15]. This association might be considered as adventitious with FMF while 10% of kidney biopsy presents IgA deposits in asymptomatic patients. Nevertheless, this patient had a full symptomatic IgAN with proliferation lesions on kidney biopsy and abnormal biomarkers.
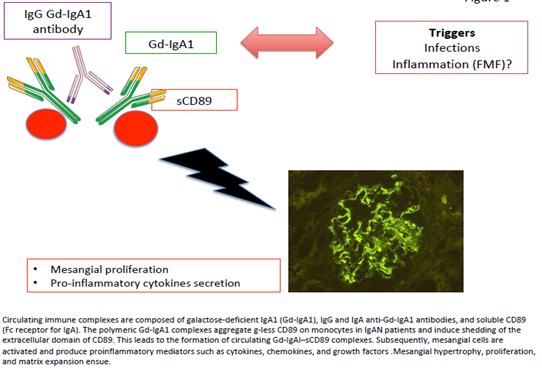
Clinical studies have attempted to demonstrate a link between clinical manifestations, renal prognosis, and the biomarkers involved in IgAN physiopathology. Berthoux et al. showed an increase in the absolute renal risk of dialysis with high levels of Gd-IgA1 and IgG anti-free O-glycoside antibodies at diagnosis [5]. From studies conducted in humans and animals [16, 17], CD89 seems to be an element that regulates disease activity and severity. This is further supported by the recent retrospective study by Berthelot et al. of a renal transplantation cohort Before kidney engraftment, the researchers were able to predict the recurrence of IgAN through serum measurement of the three key factors of CIC in IgAN: Gd-IgA1, and IgA–IgG and IgA1–sCD89 complexes [18]. This report highlighted not only the key role of these factors in the pathophysiology of IgAN but also how they might play a potent role in determining the severity and activity of the disease (Figure 1).
Our patient presented mesangial proliferation and FSGS lesions, which are associated with a higher risk of progression to chronic renal failure [19, 20]. Since the proliferative lesions were limited on our patient’s kidney biopsy, he was only treated by ISRA and colchicine and presented a complete remission of proteinuria at the end of follow up. IgAN is multi- hit pathogenesis mode. The physiopathology between FMF and IgAN is not understood. Pharyngitis infections are known to be trigger of IgA attacks. It could be suggested that inflammation linked to the fever in FMF could also play the role of a trigger in a patient, with a susceptibility to develop IgAN due to the presence of hypogalactosylated IgA and be responsible for the formation of immune complexes with IgA anti-Gd-IgA1 antibodies, and sCD89-IgA complexes (Table 2).
|
Technical Points |
|
- Familial Mediterranean Fever (FMF) is the most common form of auto-inflammatory disease, and its main renal complication is type A Amyloidosis. - Renal biopsy should be performed when there is a renal involvement because non-amyloidotic renal complications have been described. - Diagnosis of IgA should be documented by genetics, typical clinical history, renal biopsy findings and the positivity of new circulating biomarkers - There are potential candidate biomarkers of cIgAN for non-invasive monitoring of disease Gd-IgA1, IgG and IgA anti-Gd-IgA1 antibodies, and sCD89-IgA. - The physiopathology between FMF and IgAN is not understood. The fever in FMF could be a trigger in a patient, with a susceptibility to develop IgAN due to the presence of hypogalactosylated IgA. |
Table 2: Teaching points.
Figure 1: Circulating immune complexes are composed of galactose-deficient IgA1 (Gd-IgA1), IgG and IgA anti-Gd-IgA1 antibodies, and soluble CD89 (Fc receptor for IgA). The polymeric Gd-IgA1 complexes aggregate g-less CD89 on monocytes in IgAN patients and induce shedding of the extracellular domain of CD89. This leads to the formation of circulating Gd-IgAI–sCD89 complexes. Subsequently, mesangial cells are activated and produce proinflammatory mediators such as cytokines, chemokines, and growth factors. Mesangial hypertrophy, proliferation, and matrix expansion ensue.
Statements
All papers must contain the following statements after the main body of the text and before the reference list
Acknowledgement
Contributions to research of Manuscript: Robert Debré Hopsital, Pediatric Nephrology department and Pediatric department. Laboratory of Pr Renato Monteiro INSERM U1149-Centre de Recherche sur 'Inflammation, Université Paris Diderot - Faculté de médecine site Bichat. Paris.
Statement of Ethics
Parents have given their written informed consent to publish their case.The ethics committee of St Antoine Hospital in Paris, France approved the study.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding source was given for this work.
Author Contributions
Dr Marwa CHBIHI: substantial contributions to the conception and design of the work; acquisition, analysis, and interpretation of data for the work.
Dr Alexandra CAMBIER: substantial contributions to the conception or design of the work; acquisition, analysis, or interpretation of data for the work; drafting the work and revising it critically for important intellectual content and inal approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dr Cécile DUMAINE: substantial contributions to the conception and design of the work; acquisition, analysis, or interpretation of data for the work.
Pr Georges DESCHENES: final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Pr Julien HOGAN: agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and final approval of the version to be published.
Pr Renato Monteiro:Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and final approval of the version to be published.
Dr Anne COUDERC: final approval of the version to be published.
References
- Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris) 74 (1968): 694-695.
- Shen H, Gu W, Mao J, et al. Clinical characteristics of concomitant nephrotic IgA nephropathy and minimal change disease in children. Nephron 130 (2015): 21-28.
- Robert T, Berthelot L, Cambier A, et al. Molecular Insights into the Pathogenesis of IgA Nephropathy. Trends Mol Med 21 (2015): 762-775.
- Nakata J, Suzuki Y, Suzuki H, et al. Changes in Nephritogenic Serum Galactose-Deficient IgA1 in IgA Nephropathy following Tonsillectomy and Steroid Therapy. PLoS ONE 9 (2019).
- Berthoux F, Suzuki H, Thibaudin L, et al. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol JASN 23 (2012):1579-1587.
- Said R, Hamzeh Y, Said S, et al. Spectrum of renal involvement in familial Mediterranean fever. Kidney Int 41 (1992): 414-419.
- Kucuk A, Gezer IA, Ucar R, et al. Familial Mediterranean Fever. Acta Medica Hradec Kralove Czech Repub 57 (2014): 97-104.
- Peleg H, Ben-Chetrit E. The Kidney in Familial Mediterranean Fever. J Rheumatol 40 (2013): 1948-1950.
- Heller H. Amyloidosis in Familial Mediterranean Fever: An Independent Genetically Determined Character. Arch Intern Med 107 (1961): 539.
- Ozdogan H, Arisoy N, Kasapçapur O, et al. Vasculitis in familial Mediterranean fever. J Rheumatol 24 (1997): 323-327.
- Tekin M, Yalçinkaya F, Tümer N, et al. Familial Mediterranean fever--renal involvement by diseases other than amyloid. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 14 (1999): 475-479.
- Eliakim M, Rachmilewitz M, Rosenmann E, et al. Renal manifestations in recurrent polyserositis (familial Mediterranean fever). Isr J Med Sci 6 (1970): 228-245.
- Said R, Nasrallah N, Hamzah Y, et al. IgA Nephropathy in Patients with Familial Mediterranean Fever. Am J Nephrol 8 (1988): 417-420.
- Rigante D, Federico G, Ferrara P, et al. IgA nephropathy in an Italian child with familial Mediterranean fever. Pediatr Nephrol 20 (2005): 1642-1644.
- Gok F, Sari E, Erdogan O, et al. Familial Mediterranean fever and IgA nephropathy: case report and review of the literature. Clin Nephrol 70 (2008): 62-64.
- Berthelot L, Papista C, Maciel TT, et al. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med 209 (2012): 793-806.
- Vuong MT, Hahn-Zoric M, Lundberg S, et al. Association of soluble CD89 levels with disease progression but not susceptibility in IgA nephropathy. Kidney Int 78 (2010): 1281-1287.
- Berthelot L, Robert T, Vuiblet V, et al. Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int 88 (2015): 815-822.
- Hill GS, Karoui KE, Karras A, et al. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. I. Immunohistochemical studies. Kidney Int 79 (2011): 635-642.
- Cambier A, Rabant M, Peuchmaur M, et al. Immunosuppressive Treatment in Children With IgA Nephropathy and the Clinical Value of Podocytopathic Features. Kidney Int Rep 3 (2018): 916-925.